- Every year millions of undergo LASIK eye surgery as an alternative to spectacles and contact lenses
- The FDA’s revised guidelines suggest that patients receive completely transparent information on the risks and benefits of LASIK eye surgery
Risks of Lasik Eye Surgery
“Since the time of the LASIK Advisory Committee meeting in 2008, the FDA has continued to gather new information about risks associated with LASIK, including dry eye, pain and discomfort, and visual symptoms,” said a spokesperson for the FDA in an email. “Both clinical and scientific knowledge about these adverse events and symptoms has increased since that last advisory meeting,” they said.According to the spokesperson, the FDA is suggesting that manufacturers offer physicians and patients a ‘patient decision checklist’ before a LASIK treatment to ensure that both doctors and patients have all of the information, they need to make an informed decision.
The checklist includes a list of illnesses that may put a person at risk for complications after the procedure, such as controlled and uncontrolled diabetes, dry eyes, or a weakened immune system. The guideline describes the risks of the surgery, which include permanent visual loss, double vision, retinal detachment, dry eye, and pain. It also warns about the “possible risk of psychological injury,” citing reports that some LASIK patients have had extreme depression or suicidal tendenciesity, which they feel is due to difficulties after the treatment. According to the report, no conclusive causal link between the operation and the claimed psychological problems has been established.
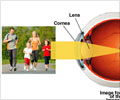
What is LASIK surgery
LASIK, or laser-assisted in situ keratomileuses, is a procedure that uses a special type of cutting laser to change the curvature of the cornea to improve vision. The outpatient operation lasts approximately 30 minutes.According to Forbes, over 500,000 people have LASIK each year, at a cost ranging from $1,000 to $4,000 per eye. Most of the time, the surgery is seen as cosmetic and hence is not covered by health or vision insurance.
Many notable athletes and celebrities have ‘gone under the laser’ to fix their vision, including LeBron James, Tiger Woods, and Kim Kardashian.
Patient Outcomes after LASIK Surgery
In July 2022, the FDA issued a lengthy draft of advice for patients. The paper was published in the Federal Register, and the public had until November 25, 2022, to comment on the plan.A large proportion of the 640 posted comments came from people who had LASIK surgery, with remarks ranging from ‘exuberantly satisfied with my decision’ to ‘it has devastated my life’. Another caller outlined several difficulties they claim they encountered as a result of the procedure, claiming that if I had been adequately informed that I could have experienced these complications, I would never have had LASIK.
The FDA is examining and assessing comments for the final advice now that the comment period has ended. “The timeline for the final guidance issuing is dependent on several factors, and we cannot provide a firm timeline at this point,” said the FDA spokesperson.
FDA Guidance on LASIK
The FDA worked with external partners, experts, and patient groups to better understand and convey the risks, and to ensure the recommendations addressed the concerns raised throughout this collaboration, according to an FDA spokesman. The entire process has lasted more than 13 years.“For example, the FDA collaborated with the National Eye Institute and the Department of Defense to conduct research as part of the LASIK Quality of Life Collaboration Project to help better understand the potential risk of problems that can occur after LASIK,” said the spokesperson.
That initiative resulted in the creation of the Patient-Reported Outcomes with LASIK (PROWL) Symptoms and Satisfaction questionnaire survey, which comprised 574 LASIK patients (divided into two groups).
Three months following surgery, 43-46% of participants experienced new visual issues, according to the researchers. According to the findings of the PROWL study, published in January 2017 in JAMA Ophthalmology, the overall satisfaction rate among the groups varied from 96-99%.
Ophthalmogist’s Views on the FDA’s Proposed Recommendations
The American Optometric Association (AOA), which represents over 48,000 optometry doctors, students, and professionals, stated that ‘patients receive completely transparent information on the risks and benefits’ of the procedure and that ‘the AOA deems this guidance to be relevant and valuable’.However, not all eye-care professionals support the planned revisions. The draft, according to critics, lacks balance: the potential benefits of the procedure (ideally, an enhanced vision that does not require glasses or contact lenses) are noted, but the majority of the 25-page document is dedicated to explaining the potential risks.
The American Society of Cataract and Refractive Surgery demanded that the FDA retract the draft because it was extremely prejudiced and misleading to potential patients about the extraordinarily high success rate of LASIK surgery.
Critics argue that a simpler LASIK patient guide would be more useful. According to the National Center for Health Data (NCHR), a non-profit think tank that reviews research on medical and other consumer products, while a more accessible and realistic portrayal of LASIK risks and benefits is a wonderful idea, this draft falls short.
According to the group, the information in the document booklet is too comprehensive and employs scientific terminology that the average consumer cannot grasp. “Few patients will read all the material about risks, contraindications, and cautions,” according to the proposed recommendations.
The group encouraged the FDA to impose the labelling (which is now voluntary) and to update their example of a LASIK Patient Checklist to include more balanced information about hazards. “This is necessary to give patients the information many have told the FDA that they wish they had known before their LASIK procedure,” read the statement.
Source-Medindia