High-dose erythropoietin treatment does not lower the risk of death or severe brain damage before 2 years of age in children who had been born at a very premature stage.
TOP INSIGHT
High-dose erythropoietin treatment does not lower the risk of death or severe brain damage before 2 years of age in children who had been born at a very premature stage.
"There was ample preclinical data to suggest that EPO would decrease neurologic damage that preterm infants suffer," she said.
However, she added, the project team does plan to follow the children in the study as they grow, because testing at 2 years of age may not reflect their later development.
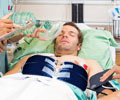
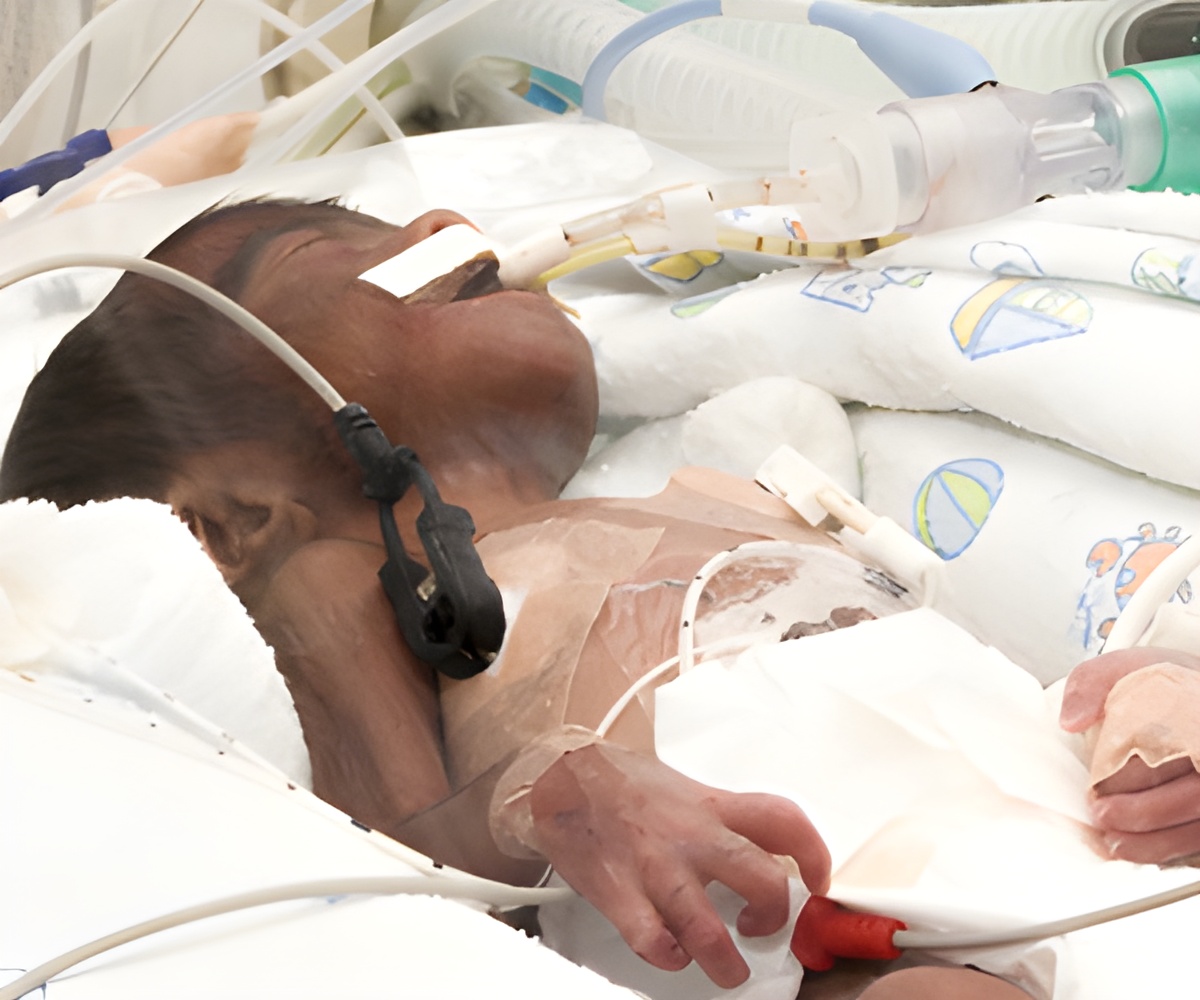
The PENUT (Preterm EPO Neuroprotection Trial) examined the effect of the drug erythropoietin (e-rith-ro-PO-e-tin, “EPO” for short) on the brains of 940 babies born between 24 and 28 weeks. They are the tiniest of preemies – babies born before 37 weeks. Started in 2013, PENUT received $10 million from the National Institutes of Health to enroll and track these babies during their hospital stays and beyond, to 2 years of age. UW Medical Center’s team are coordinating the study across 29 hospitals in the United States.
The drug was administered to newborns within 24 hours of birth. The babies then received a total of 10 injections between birth and 32 weeks. Cognitive and neurological tests are then conducted at 2 years.
Source-Newswise
 MEDINDIA
MEDINDIA


 Email
Email