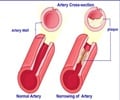
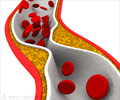
Repatha provides another treatment option for patients with hypercholesterolemia who have not been able to lower their LDL cholesterol enough with statins.

John Jenkins, director of the Office of New Drugs at the Center for Drug Evaluation and Research, said, "Repatha provides another treatment option in this new class of drugs for patients with familial hypercholesterolemia or with known cardiovascular disease who have not been able to lower their LDL cholesterol enough with statins."
One study on Repatha revealed that patients taking it for 12 weeks saw a 60% reduction in LDL levels, compared to patients taking a placebo. Side effects of Repatha may include upper respiratory tract infection, flu, back pain and reactions such as redness, pain or bruising where the injection is given. The FDA also warned that some patients may have allergic reactions.
An advisory panel to the FDA recommended the drug's approval in June, 2015. Data on whether the new drug actually leads to longer lives or less heart disease will not be available until 2017. Pharmaceutical companies suggest that the drugs could help between eight and 11 million Americans.
Source-AFP
 MEDINDIA
MEDINDIA


 Email
Email






