Prg4+ cells rapidly respond to skeletal injuries, migrating from muscle to fractures and aiding in early bone repair.
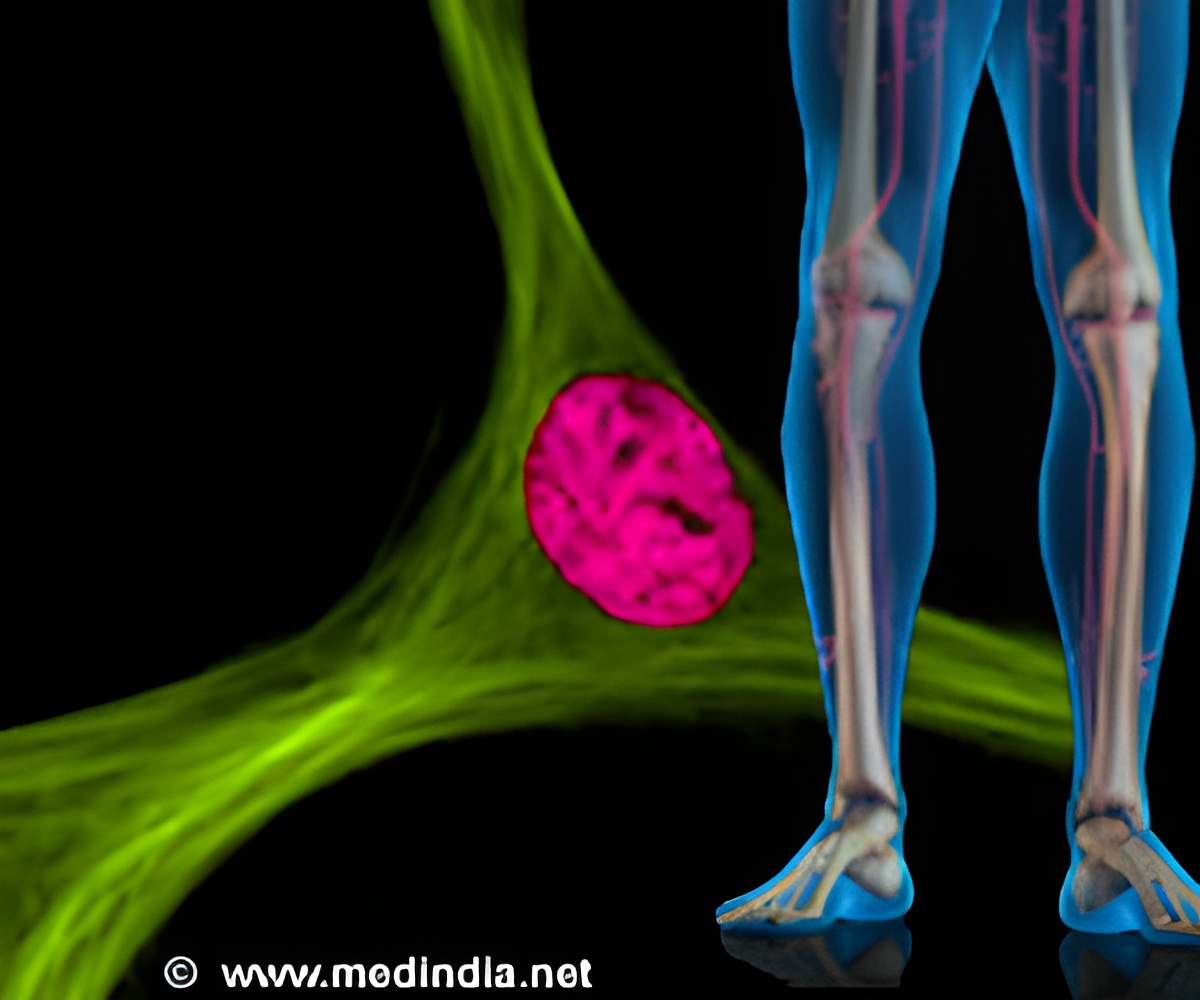
Prg4+ fibroadipogenic progenitors in muscle are crucial for bone fracture repair
Go to source). According to a new study led by scientists at the Perelman School of Medicine at the University of Pennsylvania, these muscle-derived stem cells can generate all the cell types required for bone repair, potentially offering a powerful new approach to treating complex fractures. The findings were published this week in the Proceedings of the National Academy of Sciences (PNAS).
TOP INSIGHT
Could a single type of #stemcell change how we heal from injuries? Prg4+ stem cells shows incredible promise for treating severe trauma like #open_fractures, and even everyday injuries. This is a huge step forward for #regenerative medicine! #BoneHealth #Fractures
Prg4+ Stem Cells Found to Transform Muscle into Bone
In mouse models, researchers showed that Prg4+—a type of stem cell that originates in the muscles that support the skeleton—was crucial to bone repairs—because the cells could actually transform from muscle cells to bone cells.Moving forward, the researchers believe that they could either stimulate the activity of Prg4+ cells within someone’s own body via growth factor or small molecule-based medicines, or even introduce the activated form of these cells directly to the fracture site to accelerate the bone healing.
The widely-held view is that fractured bone is mainly repaired by stem cells in the periosteum, the membrane that covers all bones. However, this repair doesn’t always work, often in the cases of “open fractures,” when a broken bone breaks the skin and often includes a huge loss of soft tissues. Why this happens is not totally understood.
Qin, her co-author Jaimo Ahn, PhD, a professor of Orthopedics at Emory University and a former Penn Medicine researcher, and their colleagues discovered that Prg4+ was a type of fibro-adipogenic progenitor (FAP), a known type of stem cell originating in skeletal muscle.
Prg4+ responded quickly to skeletal injuries, first migrating to the fracture from skeletal muscle. After that, Prg4+ was observed producing all the types of cells necessary to repair a bone— chondrocytes, osteoblasts, and osteocytes—in between the bone callus (the temporary structure formed on the bone to guide healing) and skeletal muscle.
To further prove the importance of Prg4+, when the researchers purposely destroyed Prg4+ cells, it significantly slowed healing and repair activity.
How Muscles Next to Bones Hold the Key to Better Fracture Healing
Currently, most treatment practices for fractures focus on healing bone tissue itself. But Qin, Ahn, and their colleagues have shown that extra emphasis placed on the muscles next to bones holds significant keys to injury healing.While their research holds promise for catastrophic injuries like open fractures, these findings could also play a significant role in more routine injuries.
“This could have a real impact in areas where muscles are simply just not as prevalent, like the knee and ankle,” Ahn explained. “There’s also potentially a significant impact on older adults whose muscle mass diminishes naturally, and healing doesn’t occur like it once did.”
Future research, Qin said, will build on the current findings by diving deeper into the repair abilities of other fibro-adipogenic progenitor (FAP) stem cells.
Reference:
- Prg4+ fibroadipogenic progenitors in muscle are crucial for bone fracture repair - (https://www.pnas.org/doi/10.1073/pnas.2417806122)
Source-Eurekalert
 MEDINDIA
MEDINDIA



 Email
Email