Kawasaki Disease (KD) is often mistaken for an inconsequential viral infection.
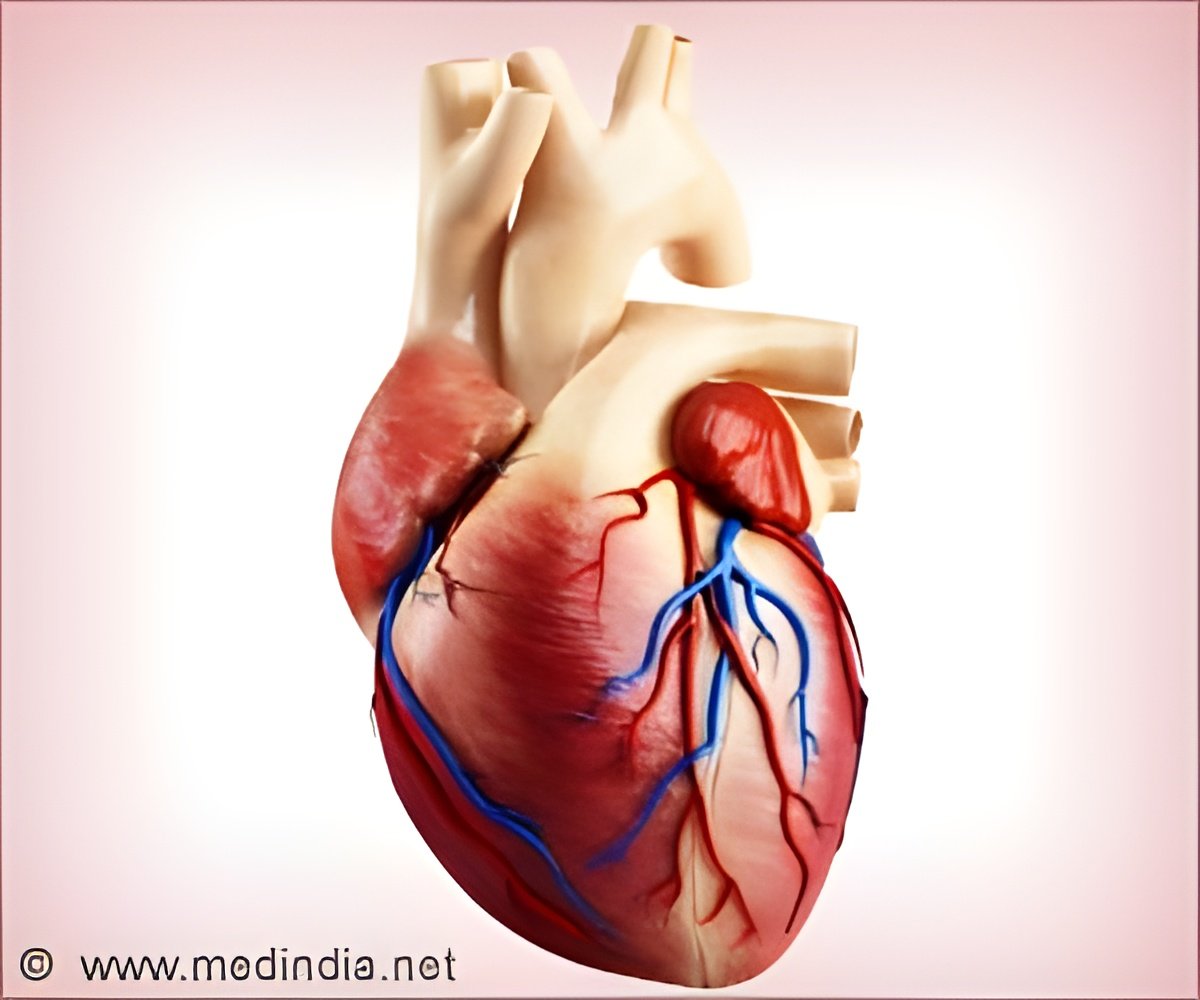
Between 10 and 20 percent of patients with KD experience fever relapse following the standard therapy with a single infusion of intravenous immunoglobulin (IVIG) and aspirin. It is known that IVIG resistance increases the risk of heart damage, most commonly a ballooning of the coronary arteries called aneurysms. These children require additional therapy to interrupt the inflammatory process that can lead to damage of the coronary arteries.
A study led by physicians at the University of California, San Diego School of Medicine and Rady Children's Hospital-San Diego looked at intensification of initial therapy for all children with KD in order to prevent IVIG-resistance and associated coronary artery abnormalities by assessing the addition of the medication infliximab to current standard therapy. The results of their study will be published in the February 24, 2014 online issue of the medical journal Lancet.
Tumor necrosis factor &alpha (TNF&alpha) is a molecule made by the body that plays a role in the development of inflammation in KD; therefore, treatment with a TNFa antagonist is a logical therapeutic intervention, according to the researchers. Early experience with infliximab - a monoclonal antibody that binds TNFa - showed promising results. A Phase 1 trial in children with KD and persistent fever following standard therapy found no infusion reactions or serious adverse events, and subsequent studies suggested that infliximab led to faster resolution of fever and fewer days of hospitalization than a second IVIG infusion.
The UC San Diego researchers conducted a trial of 196 subjects at two centers - Rady Children's Hospital-San Diego, a research affiliate of UC San Diego School of Medicine, and Nationwide Children's Hospital in Columbus, Ohio - to assess whether infliximab could reduce IVIG treatment resistance.
"While the addition of infliximab to primary treatment in acute KD did not reduce treatment resistance, it was safe and well-tolerated, achieved a greater reduction in the size of the left coronary artery, and reduced the number of days of fever and laboratory markers of inflammation," said the study's first author, Adriana H. Tremoulet, MD, of the UC San Diego Department of Pediatrics and the UC San Diego/Rady Children's Hospital-San Diego Kawasaki Disease Research Center. "We conclude that use of infliximab is safe in infants and children and that early treatment could help children with Kawasaki Disease with high levels of inflammation or early signs of coronary artery damage."
Funding for the study was provided by the U.S. Food and Drug Administration, Robert Wood Johnson Foundation, and Janssen Biotech, Inc.
Source-Newswise
 MEDINDIA
MEDINDIA


 Email
Email





