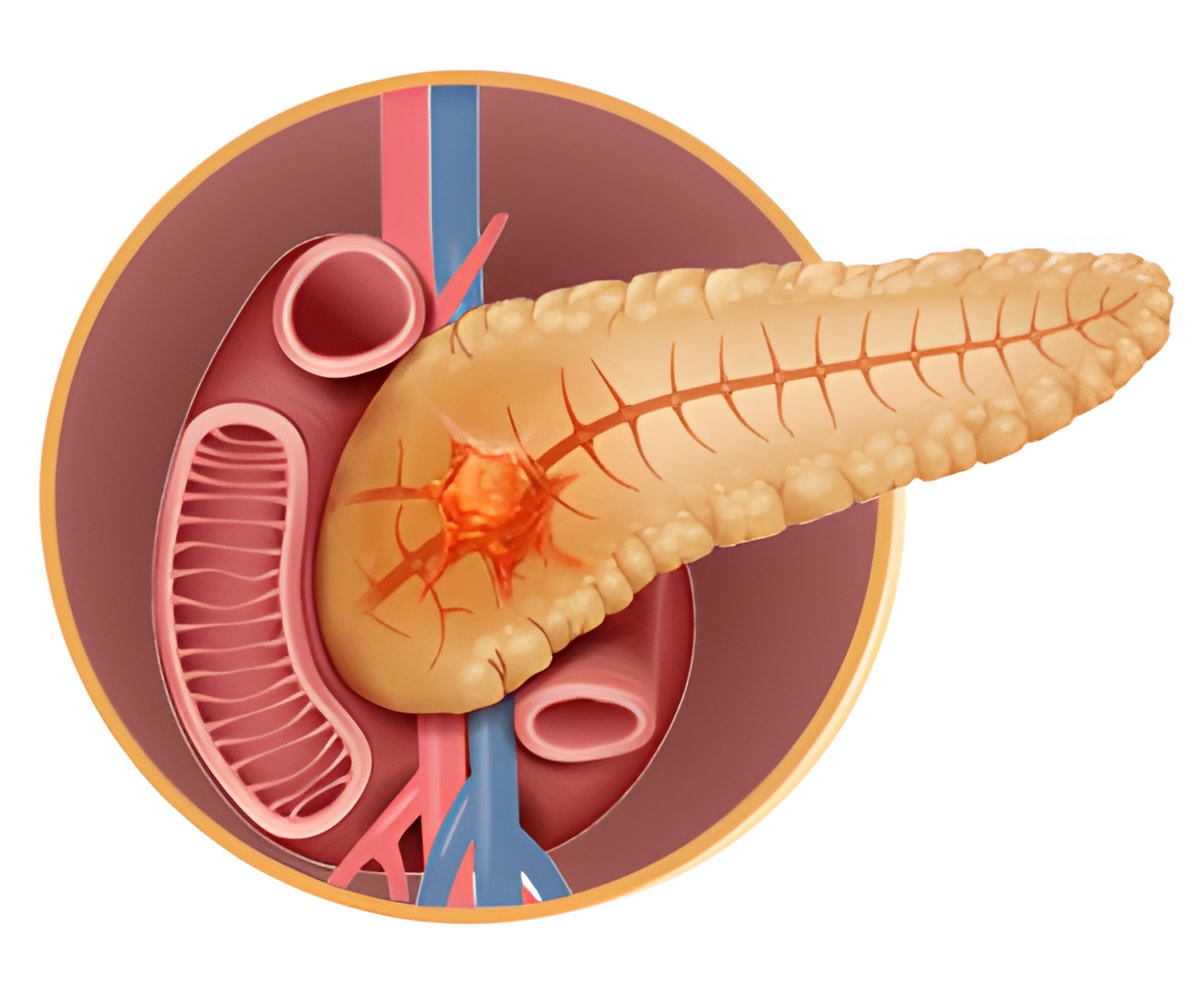
A novel mechanism has been identified that is used by the pancreatic cancer cells to disarm macrophages, which attack immune cells.
TOP INSIGHT
GDF-15 released from pancreatic tumor cells inhibits NF-kB activity in macrophages that blocks them from killing tumor cells.
When macrophages take up Gdf-15, it prevents them making nitric oxide and tumor necrosis factor, two chemicals they release to kill cancer cells. Ironically, the researchers found that NF-kB in macrophages plays an important role in the production of nitric oxide and tumor necrosis factor.
The findings are reported in the Journal of Clinical Investigation.
"This study shows that GDF-15 plays an important role in the development of pancreatic cancer and might be required for the development of early pancreatic tumors," says principal investigator Denis Guttridge, PhD, professor of Cancer Biology and Genetics at Ohio State and associate director for basic research.
"We believe that this mechanism for disarming macrophages develops early in cancer cells, enabling small tumors to survive and grow," adds Guttridge, who also is in the OSUCCC - James Molecular Carcinogenesis and Chemoprevention Program.
Guttridge and his colleagues conducted the study using pancreatic-cancer cell lines, cells from patient tumors and an animal model.
- NF-kB is a direct regulator of Gdf-15
- GDF-15 suppresses macrophage cell-killing activity by inhibiting the production of nitric oxide and tumor necrosis factor
- Both GDF-15 and NF-kB are overexpressed in patients with pancreatic cancer
"Overall," Guttridge says, "our results reveal that NF-kB is responsible for the synthesis and secretion of GDF-15 from pancreatic tumor cells, and that GDF-15 then inhibits NF-kB activity in macrophages and blocks them from killing tumor cells."
Source-Eurekalert
 MEDINDIA
MEDINDIA
 Email
Email





