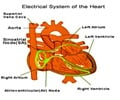
A new heart defibrillator that avoids unnecessary shocks, designed by the well-known medical device manufacturer, Medtronic has been approved by the FDA.

This implantable heart device that uses updated programming to monitor the heart’s rhythm limits the shocks to life-threatening situations. The company states that this new device will keep patients free from unnecessary shocks five years after implantation.
Although the FDA had issued a warning letter against Protecta because of quality control issues, the warning has now been lifted. Medtronic has faced other problems too, involving sales and marketing and retrenchment.
The FDA approval has been much-sought and Pat Mackin, president of Medtronic's heart rhythm unit says, "The Protecta family of devices addresses one of physicians' top needs -- allowing them to better serve patients by providing devices that are designed to deliver a shock only when needed to save a life."
Source-Medindia