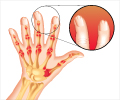
New drug upadacitinib drug showed improvements in joint swelling in patients with Rheumatoid Arthritis, an inflammatory condition that affects joints and tendons and is characterized by increased disease activity.
TOP INSIGHT
New drug for rheumatoid arthritis upadacitinib successfully completed Phase III clinical trial. The drug shows improvement in patients with an inadequate response to 'conventional synthetic disease-modifying antirheumatic drugs'.
Upadacitinib is a selective inhibitor of the enzyme Janus kinase 1 (JAK1) and has been shown to be efficacious in this patient group in earlier phase II clinical trials.
By inhibiting JAK1, upadacitinib disrupts an important signaling pathway that is responsible for triggering inflammatory responses.
In the phase III study presented, patients treated with upadacitinib showed significant improvements in joint swelling when compared to patients receiving placebo.
Patients also experienced less pain and showed improvements in joint function. Prof. Burmester is very pleased to see this new tablet-based treatment produce such significant improvements in clinical symptoms.
JAK inhibitors could help these patients achieve a quick response to treatment, allowing them to gain control over their illness.
Source-Eurekalert
 MEDINDIA
MEDINDIA



 Email
Email