New cancer research model allows researchers to arrive at findings more quickly, to conduct multiple clinical trials of pharmaceutical company drugs across several cancer types, to lessen expenses.
‘New cancer research model allows researchers to arrive at findings more quickly, to conduct multiple clinical trials of pharmaceutical company drugs across several cancer types, to lessen expenses and to increase the likelihood of finding medical solutions more quickly.’





MD Anderson has more than 50 research partnerships and alliances with pharmaceutical companies, managed through its Strategic Industry Ventures group.
Hagop Kantarjian, M.D., chair of Leukemia and co-author, Ferran Prat, Ph.D., J.D., senior vice president for Research Administration and Industry Ventures, believe the new research model addresses these needs.
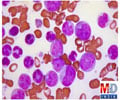
"This cancer research model is flexible and modifiable according to existing needs because it does not pretend to create a 'one-size-fits-all' approach," said Kantarjian. "These types of alliances have significant variations that accommodate the partnering drug company - its drug pipeline, research needs, financial benefits and other considerations." MD Anderson's collaboration with BMS led to simultaneous trials using combinations of immunotherapies and other treatments for several leukemia types including chronic lymphocytic leukemia, acute myeloid leukemia, chronic myeloid leukemia, and myelodysplastic syndrome. The trials include the first "triplet" immunotherapy study for leukemia, with results for all trials being analyzed by MD Anderson's top immunotherapy researchers. The collaboration has already led to the establishment of a new standard of care for treatment of chronic myeloid leukemia with an altered dose of the chemotherapy dasatinib, which has proved more effective and less toxic. The BMS collaboration has resulted in unique study approaches including:
Allowing MD Anderson leukemia researchers exploration of BMS' immune-oncology pipeline across multiple blood malignancies. Providing high-risk patients, typically excluded from BMS-sponsored studies due to CRO restrictions, access to trials at the discretion of leukemia experts. Granting a set level of funding for all trials tied to MD Anderson research programs with BMS without the need to negotiate on a trial-by-trial basis "The success of this initiative has resulted in program expansion in a number of directions," said Prat. "BMS extended the program to other clinical and research departments at MD Anderson, and is partnering with other academic cancer treatment institutions. Our Leukemia department and other MD Anderson programs have also established similar alliances with leading drug industry partners."
Source-Eurekalert