John Hopkins scientists have identified the navigation system of nerve cells.
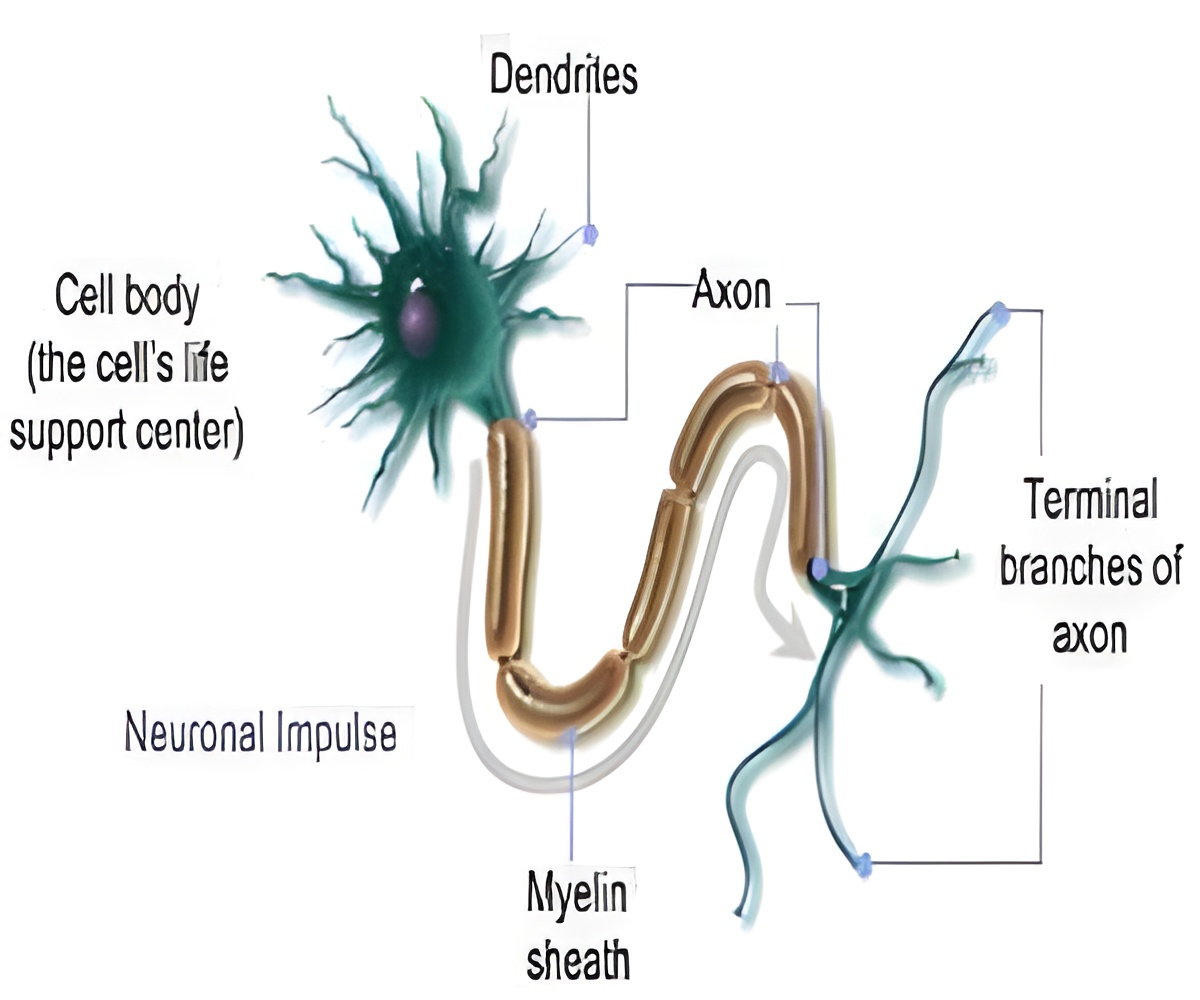
Using embryonic flies, some native (normal) and others genetically altered to lack a member of the semaphorin gene family or the receptor that binds to the semaphorin and signals within the responding neuron, the team labeled particular classes of neurons and then observed them at high resolution using various microscopy strategies to compare their axon projections.
In the native developing flies, the team saw how certain related semaphorins, proteins that nerve cells secrete into the intracellular space, work through binding their plexin receptor. First, a semaphorin-plexin pair attracts a certain class of extending neurons in the embryonic fly central nervous system assemble a specific set of target projections. Then, a related semaphorin that binds to that same plexin receptor repels these same neurons so as to position them correctly with in the central nervous system. Finally, the attractive semaphorin/plexin interaction assures the establishment of precise connections between these central nervous system axons and sensory neurons that convey messages about the external environment by extending their axons into the CNS from the periphery and contacting the assembled CNS pathways. Flies lacking this semaphorin/plexin signaling showed defects in these connections, which the researchers were able to reverse when these cues and receptors were re-introduced into flies lacking them.
To investigate whether the absence of semaphorin in flies had behavioral consequences, the team collaborated with investigators at Janelia Farm laboratories of the Howard Hughes Medical Institute and used specialized computer software to follow the movements of hundreds of fly larvae crawling on a small dish. The plate was perched on a large speaker that vibrated with pulses of sound, letting the team compare the movements of normal larvae to mutants missing semaphorin.
The "tracking" software measures differences in normal foraging behavior (mostly crawling straight and occasionally making turns) when a sound is activated. The larvae with intact semaphorin/plexin responded to sound stimulation by stopping, contracting and turning their heads from side to side. The semaphorin mutants failed to respond to the same stimuli. The researchers repeated the experiment using mutant larvae missing the protein to which semaphorin binds its plexin receptor and these larvae also showed no reaction to sound-vibration.
"The fly larvae sensory neurons, located on the larval body wall, send axon projections that do not make contact with their appropriate targets in the central nervous system when semaphorin/plexin signaling is absent," Kolodkin says. "This tells us that semaphorin cues guide not only neuronal processes assembly in the central nervous system, but also incoming projections from sensory neurons to the CNS targets."
To demonstrate that semaphorins are necessary for neuronal projections from distinct classes of neurons to make their way to correct layers in this retinal "sandwich," the scientists examined the retinas of 3-, 7- and 10-day-old mice that were genetically modified to lack either a member of the semaphorin gene family or its appropriate plexin receptor. These mutants showed severe connectivity defects in one specific inner plexiform layer, revealing faulty neuronal targeting.
"This work begins to tell us how, in a very small but highly ordered region of the nervous system, select target innervation and specific synaptic contacts between different classes of neurons can be established in the context of evolving circuit complexity" Kolodkin says.
Source-Eurekalert
 MEDINDIA
MEDINDIA


 Email
Email






