Initial experimental treatment arm results from NRG oncology trial suggests total neoadjuvant therapy for locally advanced rectal cancer is safe
‘TNT can be safely and successfully delivered to patients with locally advanced rectal cancer, which ensures that all patients get the treatment they need and gives many opportunities to improve care further.’
Read More..




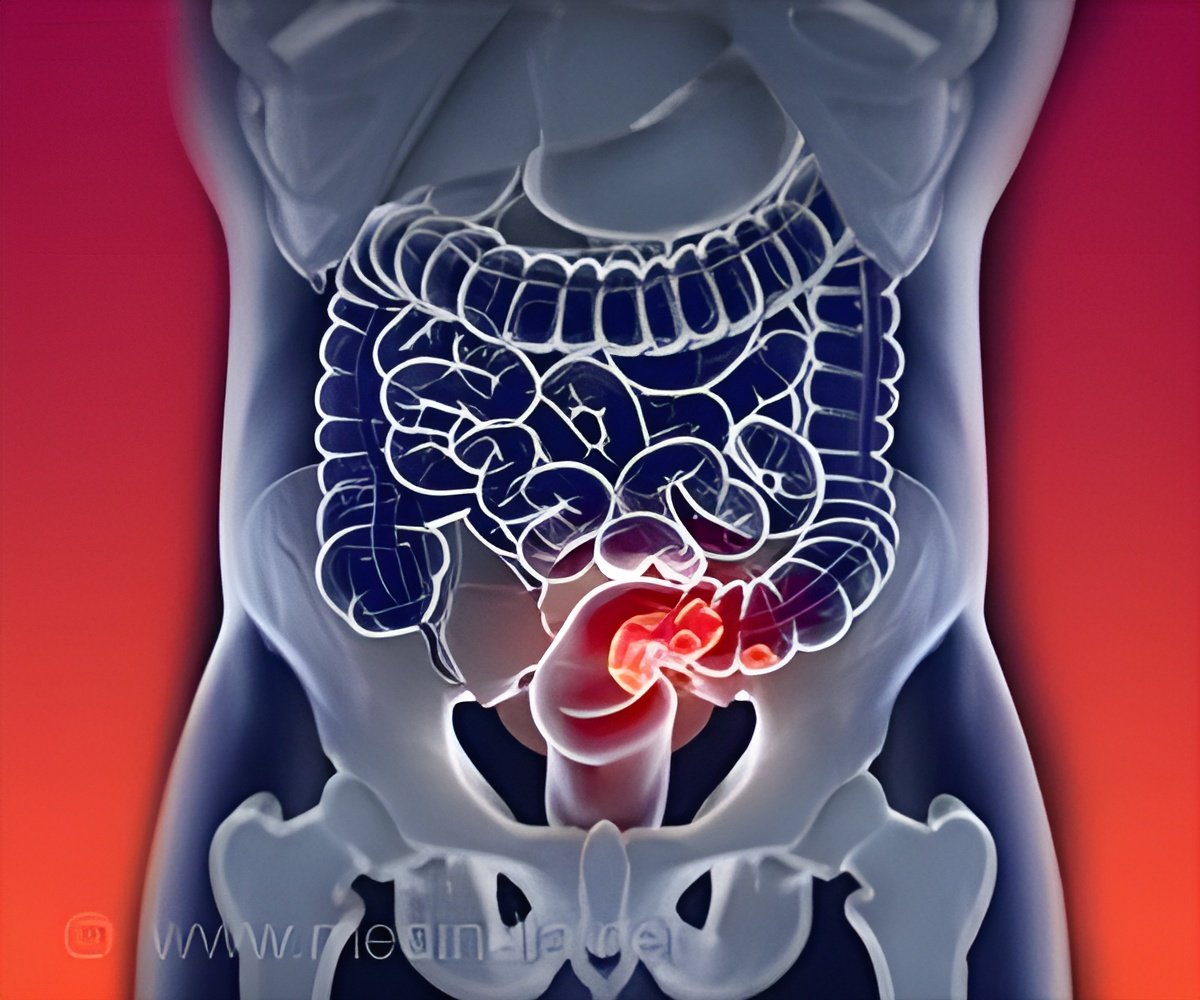
Veliparib usage as part of total neoadjuvant therapy (induction chemotherapy followed by chemoradiotherapy and surgery) in patients with locally advanced rectal adenocarcinoma on the NRG Oncology Phase II clinical trial NRG-GI002 were recently presented at the American Society of Clinical Oncology (ASCO) Annual Meeting. Results were reported on the primary endpoint of pathological regression via the neoadjuvant rectal cancer (NAR) score, a short-term clinical trial surrogate endpoint.Read More..
After treatment with a combination chemotherapy regimen called mFOLFOX6, researchers combined veliparib, a poly (ADP-ribose) polymerase (PARP) inhibitor or a drug that kills cancer cells by blocking a protein called PARP, with another chemotherapy drug named capecitabine, and radiotherapy in patients who had locally advanced Stages 2 or 3 rectal cancer.
Patients on the control arm got the same therapy with the omission of veliparib. In the first experimental arm of NRG-GI002, veliparib was added to radiotherapy and chemotherapy treatment regimens.
The primary endpoint of the mean Neoadjuvant Rectal (NAR) Score (lower number reflects more downstaging) was 12.6 for the control arm and 13.7 for the experimental arm (p=0.69). There were numerically more patients who achieved a pCR with veliparib, though (22% vs. 34%; p=0.14). This first experimental arm results demonstrated that the addition of veliparib was associated with more short-term side effects and reduced completion of chemoradiation (85% vs. 70%; p=0.026).
The most common, grade 3 and 4 side effects that were experienced on the first NRG-GI002 experimental arm included diarrhea and cytopenias. Importantly, these side effects did not appear to negatively impact short term patient outcomes.
Advertisement
Our randomized phase II trial combined 400 mg PO BID veliparib with chemoRT followed by surgery to observe improvement in patients' NAR score that would translate into improved disease free and overall survival. Unfortunately, we did not see an improvement with the use of veliparib. However and very importantly, we have now established that TNT can be safely and successfully delivered to patients with locally advanced rectal cancer. This ensures all patients get the treatments they need and gives us many opportunities to further improve care for this group of patients."
Advertisement
An ongoing second experimental arm is observing the impact of the monoclonal antibody pembrolizumab in combination with capecitabine and radiotherapy after induction mFOLFOX6 to determine if immunotherapy is also a safe, effective option for treating locally advanced rectal cancer. This second arm has completed enrollment with results eagerly awaited. Additional arms are in active development with the goal of improving outcomes for patients with this disease.
Source-Eurekalert