Addition of the novel the novel MM-398 to the standard treatment may improve survival for metastatic cancer patients, a team of researchers have found
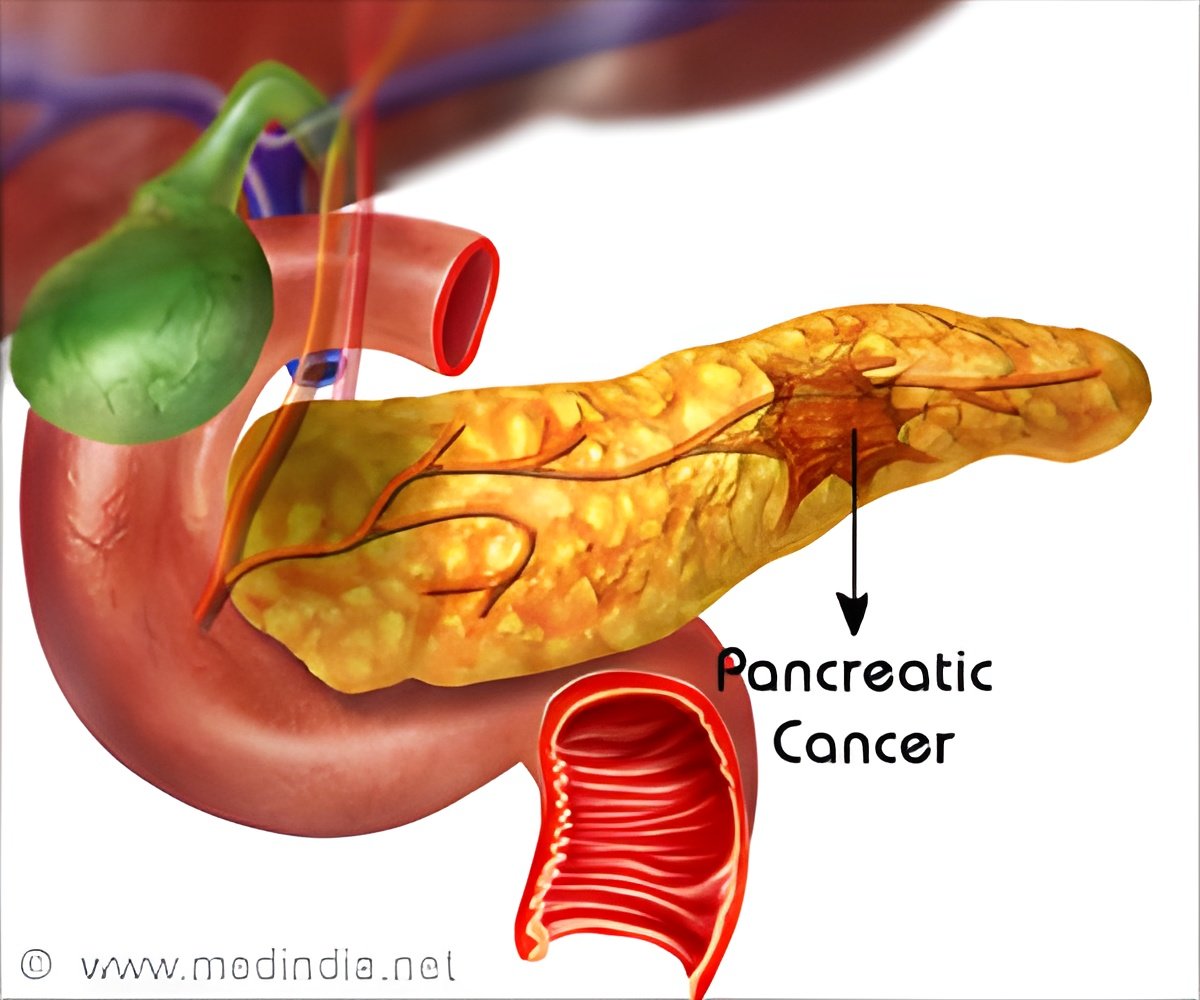
One of the biggest challenges in pancreatic cancer is drug delivery. "MM-398 (nal-IRI) is a nanoliposomal irinotecan: this delivery system allows longer drug exposure in the circulation and more accumulation of the drug and its active metabolite SN38 at the tumour site," Wang-Gillam said. "MM-398 therefore generates higher anti-tumour activity and is more effective than conventional irinotecan alone in the preclinical setting."
The phase II study had demonstrated the anti-tumour activity of MM-398 monotherapy as second-line treatment in patients with metastatic pancreatic cancer refractory to gemcitabine [1].
The current NAPOLI-1 trial was a global randomised phase III trial at more than 100 sites. There were 3 treatment arms: MM-398, standard treatment with 5-fluorouracil (5FU)/leucovorin, and MM-398 plus 5FU/leucovorin. The trial included 417 patients who had progressed or received prior gemcitabine-based therapy. The primary endpoint was overall survival.
Overall survival was significantly improved with the combination therapy of MM-398 plus 5FU/leucovorin compared to 5FU/leucovorin alone. Median overall survival was 6.1 months in the MM-398 plus 5FU/leucovorin group compared to 4.2 months in the group receiving standard treatment with 5FU/leucovorin alone (hazard ratio [HR]=0.67, p=0.012). Progression-free survival also improved significantly, from 1.5 months with the standard therapy to 3.1 months in patients receiving MM-398 plus 5FU/leucovorin (HR=0.56, p<0.001). MM-398 alone did not provide any additional survival benefit over standard therapy.
Wang-Gillam said: "The results are very exciting because the trial met its primary endpoint and found a highly statistically significant benefit of MM-398 plus 5FU/leucovorin on overall survival and progression-free survival compared to 5FU/leucovorin alone. We also found a significant benefit of the combination therapy on overall response rate and biochemical response."
Wang-Gillam concluded: "Our results are indicative of a successful effort in developing new drugs toward pancreatic cancer. We now have another viable option in this devastating disease."
Source-Eurekalert
 MEDINDIA
MEDINDIA
 Email
Email







