GSK’s phase III trials of the B-lymphocyte stimulator reduced disease activity by 61% and AZ’s phase II trials of anifrolumab reduced the activity by 51.5%.

GSK reported new phase III data on a new subcutaneous formulation of its market-leading Benlysta (belimumab) product that is the first new therapy for the disease in five decades.
In the phase III trial, a 200mg subcutaneous dose of the B-lymphocyte stimulator (BLyS) inhibitor given once a week was found to be more effective in reducing disease activity compared to placebo.
After a year, 61% of the Benlysta group showed reduced disease activity as measured by the SLE Responder Index (SRI) - a standard measure if efficacy - compared to 48% of patients on placebo.
"On the basis of these data, we expect to progress towards global regulatory filings for a belimumab subcutaneous formulation," commented Paul-Peter Tak, head of immuno-inflammation R&D at GSK.
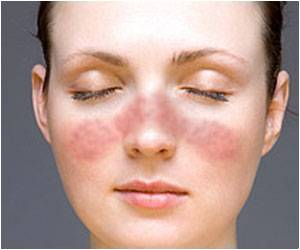
A new phase II trial conducted by AZ on the drug - called anifrolumab (formerly MEDI-546) - is an antibody blocking the type I interferon (IFN) receptor, showed that the drug significantly reduced disease activity and improved symptoms of SLE, including rash and arthritis.
"The lupus community has been disappointed too often with clinical trial results," commented principal investigator Richard Furie of North Shore-LIJ Health System. "We have been eagerly awaiting clinical data of this magnitude for many years," he added.
 MEDINDIA
MEDINDIA



 Email
Email