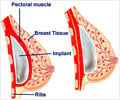
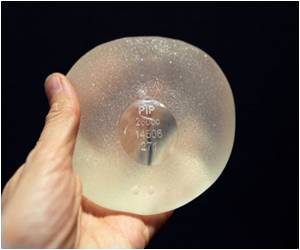
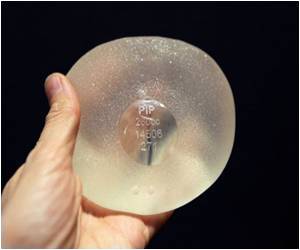
A German court began hearing on Tuesday the country's first lawsuit over a health scandal surrounding French-made breast implants found to leak silicone into women's bodies.

"With her lawsuit, the plaintiff is seeking an award primarily for pain and suffering of 20,000-30,000 euros ($25,000-38,000) from five defendants based on various legal aspects," the court said in a statement.
The plaintiff had paid 5,800 euros for implants before learning in 2010 after the scandal broke that they were filled with a sub-standard silicone gel.
The defendants include the woman's cosmetic surgeon, whom she accuses of failing to inform her sufficiently about the risks and of having promoting PIP implants in particular.
Other defendants include PIP's insurer, German inspection authorities and the German federal government, which the defendant accuses of failing in its duties to properly oversee medical products despite reports of problems.
According to federal records, more than 5,200 women in Germany received PIP implants. By mid-2012, 1,015 women had had them removed. In more than one-quarter of the cases, a rupture of the implant had been detected.
Advertisement
The first lawsuit in France is expected to be heard in April.
Advertisement
PIP was shut down and its products banned in April 2010 after it was revealed to have been using non-authorised silicone gel that caused abnormally high implant rupture rates.
Source-AFP