It re-established normal intestinal function within four days, stopped tumor growth within two weeks, and signs of cancer were prevented months later.
Senior study author Scott Lowe from Memorial Sloan Kettering Cancer Centre, New York, said, "Treatment regimes for advanced colorectal cancer involve combination chemotherapies that are toxic and largely ineffective, yet have remained the backbone of therapy over the last decade."
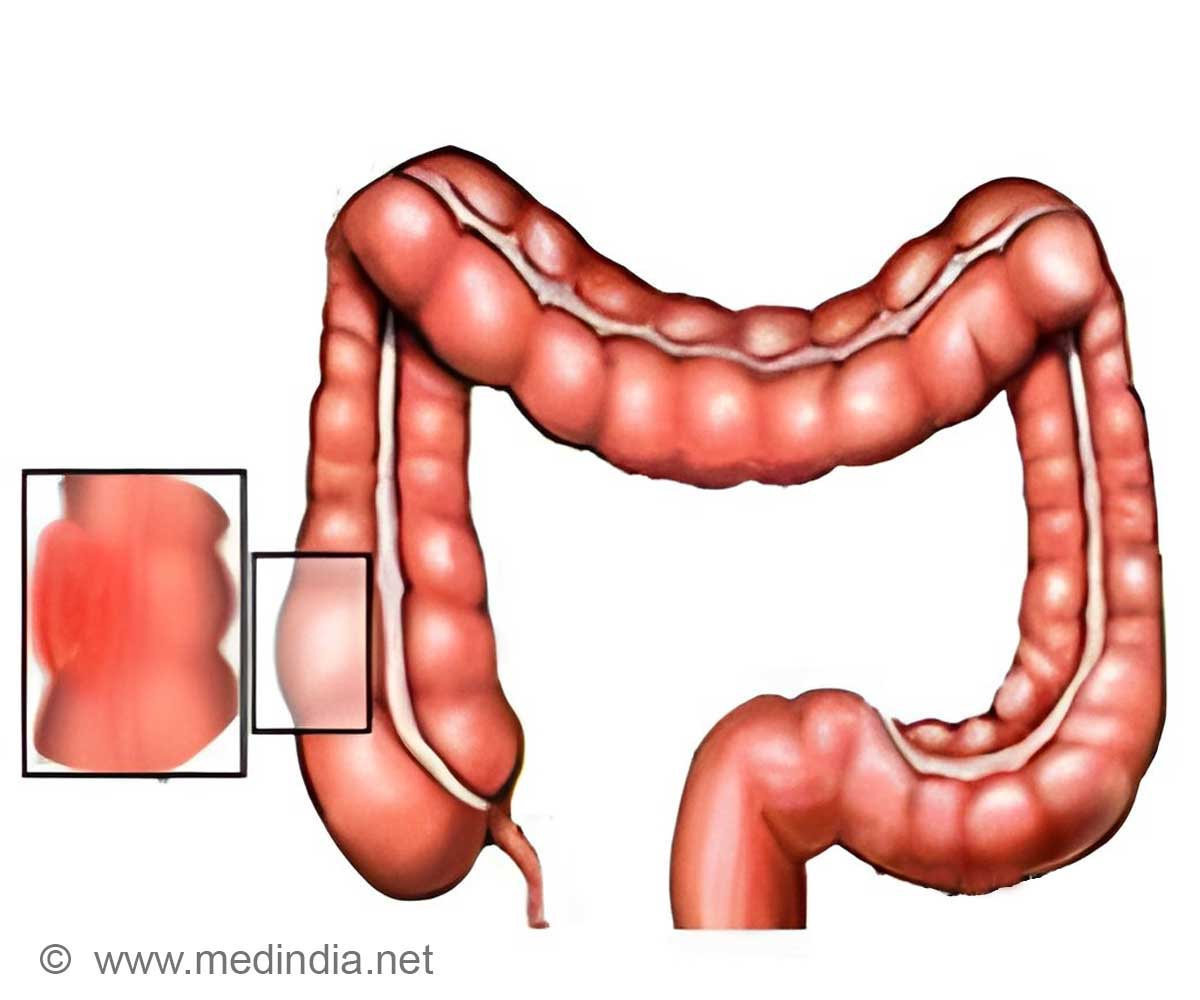
Up to 90% of colorectal tumors contain inactivating mutations in a tumor suppressor gene called adenomatous polyposis coli (Apc). Although these mutations are thought to initiate colorectal cancer, it has not been clear whether Apc inactivation also plays a role in tumor growth and survival once the cancer has already developed.
The research team used a genetic technique to precisely and reversibly disrupt Apc activity in a novel mouse model of colorectal cancer. While the vast majority of existing animal models of colorectal cancer develop tumors primarily in the small intestine, the new animal model also developed tumors in the colon, similar to clinical patients.
Consistent with previous findings, Apc suppression in the mice activated the Wnt signalling pathway, which is known to control cell proliferation, migration, and survival. When Apc was reactivated, Wnt signalling returned to normal levels, tumor cells stopped proliferating, and intestinal cells recovered their normal function. The tumors regressed and disappeared or reintegrated into normal tissue within two weeks, and there were no signs of cancer relapse over a 6-month follow-up period.
Moreover, this approach was effective in treating mice with malignant colorectal cancer tumors containing Kras and p53 mutations, which are found in about 50% of colorectal tumors in humans.
The study is published in the Cell.
 MEDINDIA
MEDINDIA
 Email
Email










