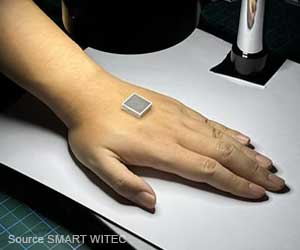
Proteus ingestible sensors embedded in smart pills can transmit data to skin-worn receiver patches that communicates to a smartphones via Bluetooth.

“We are delighted that our collaborative work with the FDA continues to enable positive progress. We believe that ingestible devices have the potential to speed clinical trials and improve the real-world effectiveness of medicines in community settings,” said Dr. George Savage, Proteus co-founder and CMO.
The tiny ingestible sensors attached to the drugs and a Proteus Patch worn on the torso detects the time of ingestion, rest and heart rate when the drugs are swallowed.
The patch uses built-in Bluetooth antenna to share when every pill was swallowed to a smartphone app. The readings can be transferred to family, caretakers and the patients’ physician.
The technology was approved by the FDA in 2012, but the ability to accurately record when every pill was ingested has only now been sanctioned by FDA to be used to measure medication adherence.
Source-Medindia
 MEDINDIA
MEDINDIA



 Email
Email