The first fully interoperable continuous glucose monitoring system (CGM) approved by the The US Food and Drug Administration (FDA) helps determine the levels of blood sugar in children and adults with diabetes.

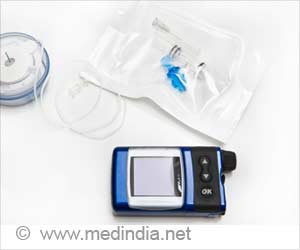
‘Dexcom G6 device contains a small sensor that helps measure the amount of glucose in body fluid continuously.’





The FDA's decision to permit marketing of the Dexcom G6 integrated continuous glucose monitoring system could streamline the review pathway for similar devices. "The ability of this device to work with different types of compatible devices gives patients the flexibility to tailor their diabetes management tools to best meet personal preferences," said Donald St. Pierre of the FDA.
Diabetes impairs the body's ability to make or properly use the blood glucose-regulating hormone insulin.
They must regularly monitor their blood sugar levels since continuously high blood sugar levels can lead to heart disease, stroke, blindness, kidney failure and nerve damage leading to amputation of the toes, feet or legs.
Blood sugar levels can also fall too low, which can cause dizziness, confusion, unconsciousness and, in extreme cases, death.
Advertisement
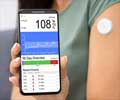
The device transmits real-time glucose readings every five minutes to a compatible display device such as a mobile medical app on a cell phone and will trigger an alarm when a patient's blood sugar enters a danger zone soaring too high or dropping too low.
Advertisement
"We listen closely to people with diabetes and continuously look for ways to empower them to better manage their condition," Dexcom CEO Kevin Sayer said in a statement after the FDA approval of the device.
"We believe the new Dexcom G6 is a significant step forward for Dexcom and our industry," Sayer said.
Source-IANS