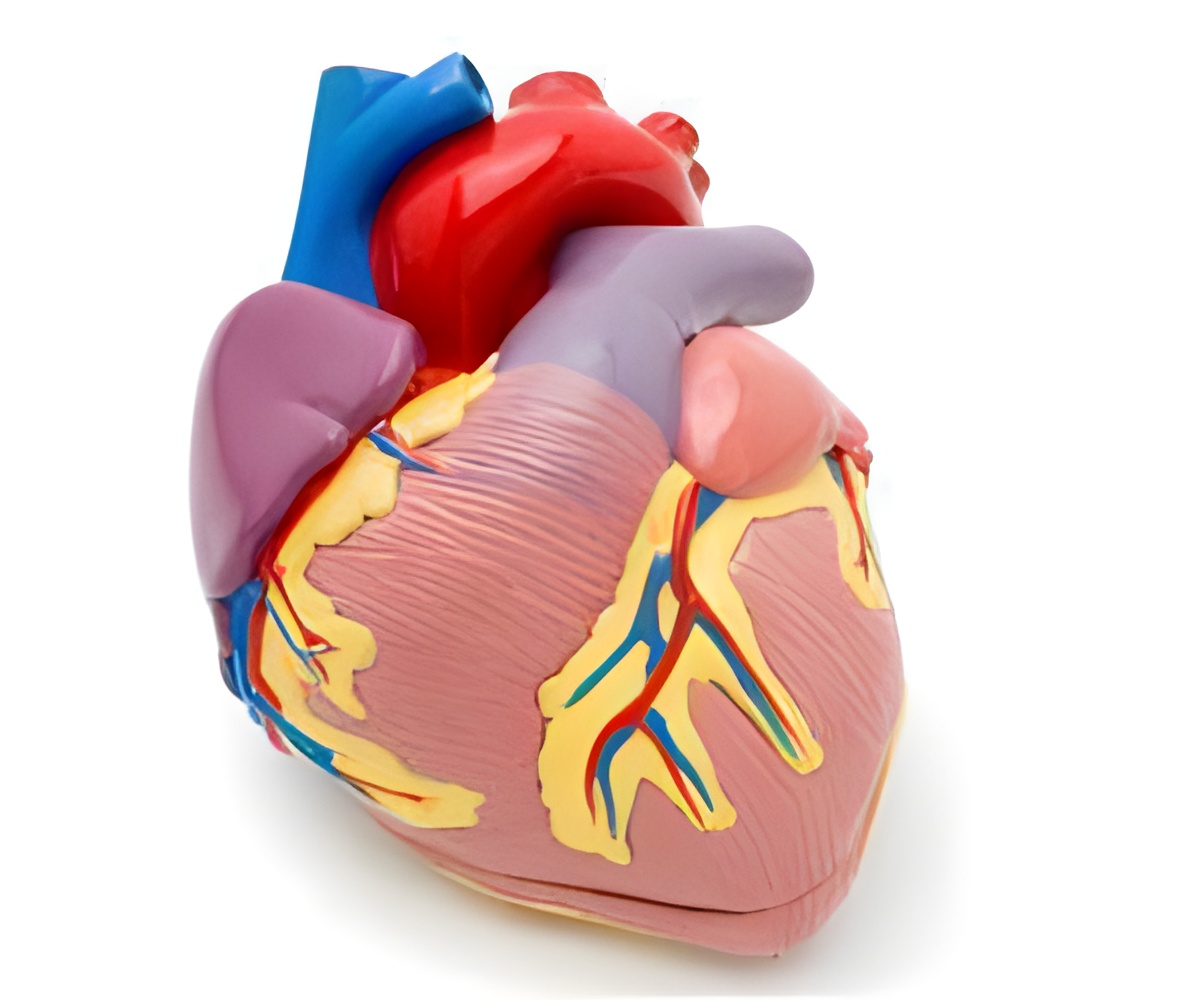
France has begun the first human trial of artificial heart, which aims at overcoming shortages of organs available for transplant.
The patient, whom it did not name, is in intensive care, "is conscious and speaking to relatives," Carmat said, adding that it was too early to draw wider conclusions about the operation.
Artificial hearts have been in use for many years as a temporary fix for patients with chronic heart problems.
Tens of thousands of people with hearts damaged by disease or a heart attack die each year because of a lack of a donor.
The Carmat product aims at providing a longer-term solution to bridge the wait and enable hospitalised patients to return home and maybe even resume work.
A self-contained unit implanted in the patient's chest, it uses soft "biomaterials" and an array of sensors, rather than a pump, to mimic the contractions of the heart.
Power comes from an external source or wearable lithium batteries.
The 900-gramme (31-ounce) device is the outcome of a years-long collaboration between cardiac surgeon Alain Carpentier and the European aerospace giant EADS.
Nearly 100,000 people in Europe and the United States are in need of a heart transplant, according to Carmat.
The price of the heart is estimated at between 140,000 and 180,000 euros ($190,000 and $244,000).
Phase I of the trial, on the small group of volunteers in terminal condition, will assess survival one month after the operation, or earlier if the patient receives a natural heart.
If all goes well, a second phase, conducted among a group of about 20 patients, will look at efficacy -- quality of life, comfort and side effects -- as well as safety.
A US rival to Carmat, an artificial heart called AbioCor made the biotech firm Abiomed, is authorised in the United States for patients with end-stage heart-failure or life expectancy of less than 30 days, who are not eligible for a natural heart plant and have no other viable treatment options.
Source-AFP
 MEDINDIA
MEDINDIA


 Email
Email