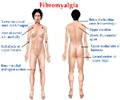
Lyrica, Cymbalta and Savella are the three drugs approved in the US and currently dominate the sales across the seven major markets and form the core treatment for fibromyalgia.
Maura Musciacco, GlobalData’s Director of Neurology and Ophthalmology, says the US will consolidate its position as the dominant market, with the country’s share increasing from 83% of fibromyalgia treatment sales in 2013 to 86% by 2023.
Musciacco explains: “The US is the only country among the 7MM with three approved brands: Lyrica, Cymbalta, and Savella. Along with its higher drug prices and prescribing rates, the largest fibromyalgia population, and a greater number of pipeline products compared with the other six countries, the US holds a greater share of the fibromyalgia treatment arena."
“The small range of currently available fibromyalgia therapeutics means that the market will welcome new products that can offer patients better efficacy, convenience, and safety. To date, four pipeline agents will potentially be introduced by 2023, mainly in the US.”
These products include two reformulations, namely Pfizer’s Lyrica CR and Tonix Pharmaceutical’s TNX-102 SL, and two “me-too” products, namely Daiichi Sankyo’s DS-5655 and Theravance Biopharma’s TD-9855.
Musciacco continues: “The estimated cost of these treatments will be comparatively higher than the currently available fibromyalgia therapies and the inexpensive generic products. These higher costs will drive an increase in the overall value of the fibromyalgia market over the forecast period."
Advertisement
Source-Medindia