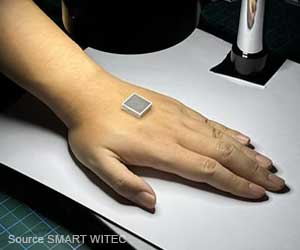
Protege MRI spinal cord stimulator is also an upgradeable MR-conditional device that can be safely used while the patient receives extremity MRI scans.
According to the company, the Protege MRI system is the smallest MR-conditional SCS implantable pulse generator (IPG) available in the United States, and the only upgradeable IPG on the market to allow patients to safely undergo head and extremity MRI scans.
Upgradeable technology allows patients to access future SCS technology from St. Jude Medical, through software updates rather than surgical device replacement. Historically, most patients would need additional surgery to receive new product features and benefits.
“The launch of the Protege MRI system provides physicians with a solution that offers the benefits of future therapy upgrades as they are approved without the need for a future surgery. The Protege MRI system is an innovative technology advancement that optimizes chronic pain care without compromising a patient’s potential need for future head and extremity MRI scans,” said Dr.Robert Levy, director of the Marcus Neuroscience Institute in Boca Raton, Florida.
Chronic pain affects more than 100 million Americans, an incidence rate which outpaces heart disease, cancer and diabetes combined. In total, the condition costs the American population 515 million workdays annually and generates upwards of 40 million visits to physicians each year.
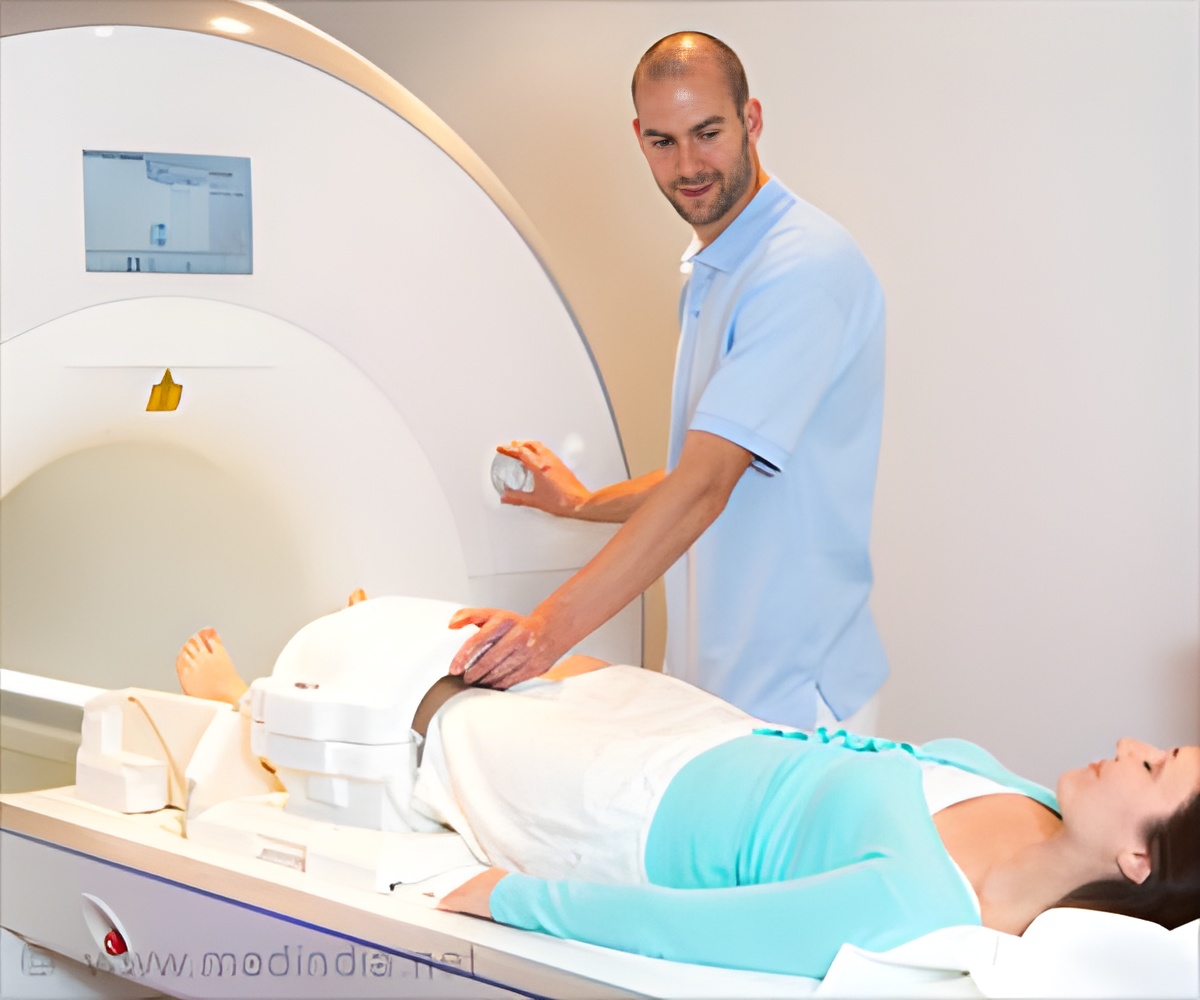
SCS therapy can offer proven, meaningful chronic pain relief for many patients while improving quality of life and reducing or even eliminating a patient’s use of pain medication. Yet for some patients battling chronic pain, the possible need for future MRI scans has acted as a barrier to SCS therapy.
Source-Medindia
 MEDINDIA
MEDINDIA
 Email
Email