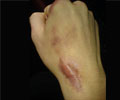
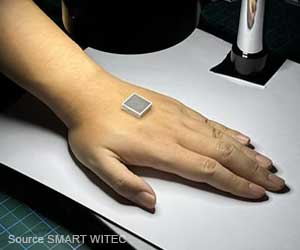
The FDA has given the nod to Sensus Healthcare (Boca Raton FL) to start the marketing of its SRT-100 superficial radiotherapy system for the treatment of keloids, or scar tissue

“Painful and potentially disfiguring keloid scars are very difficult to effectively treat by surgery or other means due to recurrence rates as high as 45 to 100%. Studies show that the use of adjunctive radiation therapy can dramatically reduce the rate of recurrences,” said Mark S. Nestor , MD, PhD of the Center for Cosmetic Enhancement in Aventura, FL.
SRT-100™ is the perfect treatment method for non-melanoma skin cancers and keloids, with its SharpBeam™ technology. This system ensures that only the targeted lesion is treated and the surrounding and underlying healthy tissue is not touched.
Source-Medindia
 MEDINDIA
MEDINDIA

 Email
Email