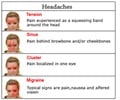
Erenumab is very useful for chronic migraine patients for whom successful prophylaxis of other drug treatment options has been exhausted.

TOP INSIGHT
Erenumab could reduce the burden of a chronic migraine among patients for whom other substances used for prophylaxis have failed or have not been an option.
Read More..
Drug class blocks CGRP transmitter
In contrast to acute treatment, there had been no specific drugs for prophylaxis of migraine so far. A range of drugs have been available for a long time, but they all have been developed for other conditions, such as hypertension, depression or epilepsy.
It has long been known that a transmitter called calcitonin gene-related peptide (CGRP) plays a key role in the development of migraine. But it took decades before a drug was developed that blocks CGRP without having major side effects.
Erenumab is the first drug of a new drug class that became ready for the market. Its mechanism of action consists in inhibiting the function of the CGRP receptor in the brain.
The Federal Joint Committee (G-BA) differentiated three groups of patients, who all have at least four migraine days per month. They were differentiated by whether the patients have received prophylactic medication and, if any, by the type of prophylactic medication.
The appropriate comparator therapy specified by the G-BA was best supportive care (BSC), i.e. the best possible therapy optimized for the individual patient, such as psychotherapy or relaxation techniques, for example.
Only patients with episodic migraine were included
The data from 193 patients used by the company in the dossier were from the randomized controlled trial LIBERTY. Over a period of 12 weeks, the study participants received either subcutaneous erenumab injections once a month or placebo, each in addition to BSC.
Eligible patients had migraine for four to 14 days per month (episodic migraine), and had been treated unsuccessfully with between two and four preventive medications. Patients with chronic migraine, who, according to the classification, have a headache for at least 15 days per month, were not included in the LIBERTY study.
Fewer migraine days, less impairment
The analysis of these data showed that notably more participants in the erenumab group than in the placebo group had a 50% or greater reduction in the number of headache days. This was also shown for the number of migraine attacks, which can last several days.
The results regarding "general impairment" from headache and "activity impairment" were also in favour of erenumab.
In contrast, there were no differences or no relevant differences regarding "physical functioning", "work productivity", health status and side effects. The outcome "quality of life" was not recorded at all in the LIBERTY study.
Number of headache days as the only criterion
Erenumab was approved for both episodic and chronic migraine. The study results also do not have to be restricted to episodic migraine as the literature provides no medical or other substantive justification for the value of "14 days" to distinguish episodic from chronic migraine. In addition, the participants in the LIBERTY study were in the transition period between episodic and chronic migraine.
Overall, IQWiG therefore sees an indication of a considerable added benefit of erenumab for the prophylaxis of migraine.
Source-Eurekalert
 MEDINDIA
MEDINDIA

 Email
Email