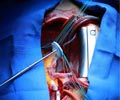
The device was designed to lower the chances of leakage around the valve, which should reduce various side-effects, including the potential for stroke.
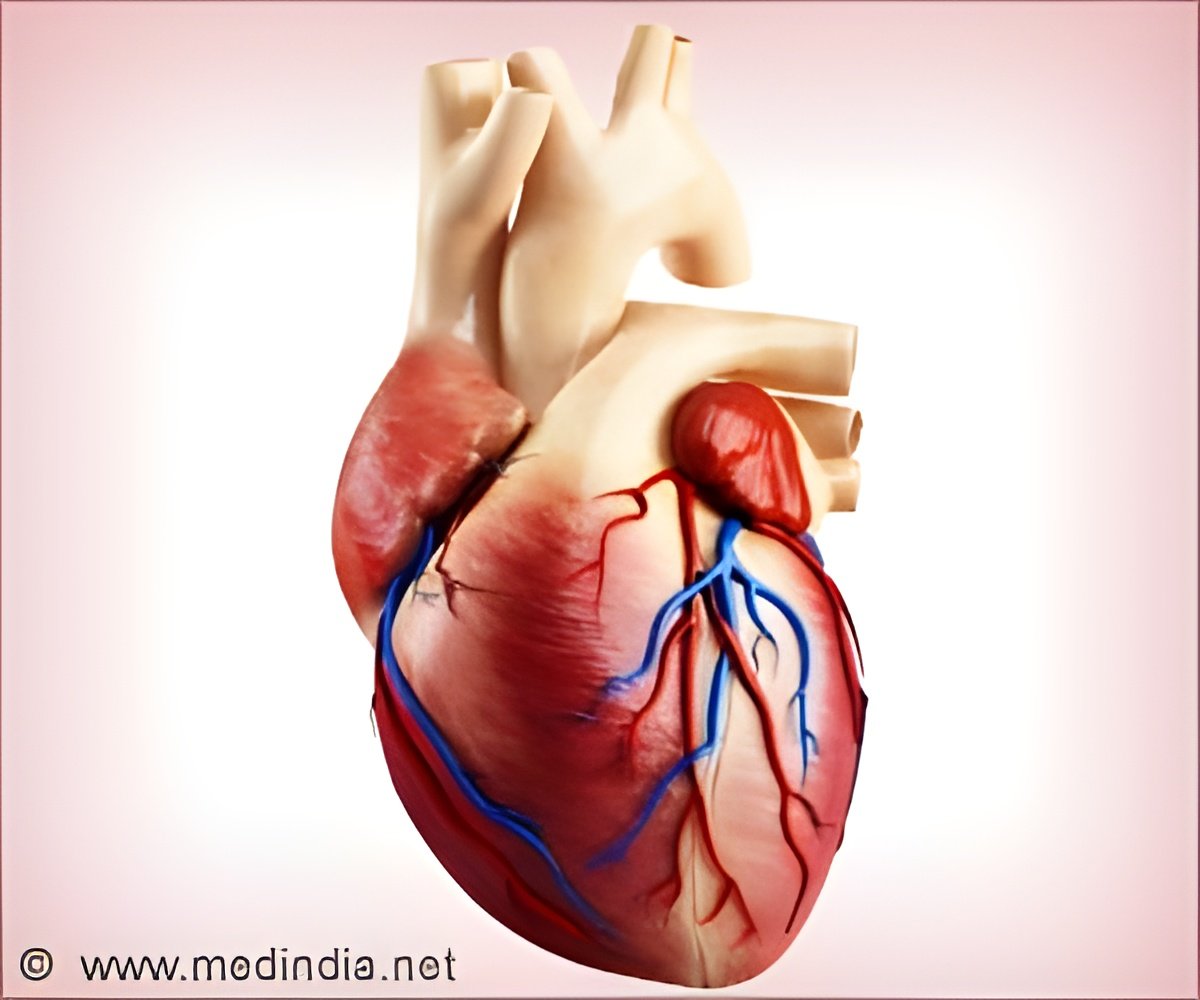
The SAPIEN 3 now has an outer layer of polyethylene terephthalate over the frame of the device that more consistently plugs blood flow from having a chance to pass through outside of the valve.
The SAPIEN 3 valve is the only commercial transcatheter heart valve, which has shown a low rate of complication. The valve can be implanted through multiple approaches: transfemoral, transapical or transaortic.
“Edwards SAPIEN 3 valve has a unique design intended to provide a simpler procedure, along with fewer post-procedural complications and a faster recovery for patients,” said Larry L. Wood, Edwards’ corporate vice president, transcatheter heart valves.
“Based on our clinical leadership in transcatheter heart valves, we believe the SAPIEN 3 valve’s characteristics and procedural refinements have the potential to transform TAVI for both physicians and patients.”
Source-Medindia
 MEDINDIA
MEDINDIA



 Email
Email