Cell therapy may improve upper limb strength and heart function in Duchenne muscular dystrophy patients
TOP INSIGHT
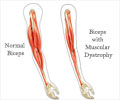
Affecting 1 in 3,600 boys, Duchenne muscular dystrophy is a neuromuscular disease caused by an absence of a key muscle protein called dystrophin, which leads to progressive muscle degeneration.
"This is the first trial to test cell therapy to treat heart disease in patients with Duchenne muscular dystrophy," said Ronald G. Victor, MD, associate director of the Cedars-Sinai Heart Institute and one of the clinical trial’s primary investigators. "These early results show that further research is warranted and, in fact, is being planned."
Affecting 1 in 3,600 boys, Duchenne muscular dystrophy is a neuromuscular disease caused by an absence of a key muscle protein called dystrophin, which leads to progressive muscle degeneration.
Most Duchenne patients lose their ability to walk between ages 12 and 15. Average life expectancy is about 25 years.
"The need is great because there is no current treatment to address heart failure in these patients," said Eduardo Marbán, MD, PhD, director of the Cedars-Sinai Heart Institute and the researcher who developed the cardiosphere-derived cell (CDC) technology used in the study.
In the study, 25 patients ages 12 to 22 were treated at the Cedars-Sinai Heart Institute, University of Florida Health or Cincinnati Children’s. Twelve patients were randomly assigned to receive standard care consisting of prescription medications.
As measured by MRI, the patients who received the progenitor cells experienced a 7 percent reduction in the area of the heart scarred by cardiomyopathy. Patients who had the usual regimen saw an increase in their heart scars. Among the subgroup of patients with the most advanced impairment of arm function, one year after treatment, eight out of nine patients who received the CDCs experienced improved skeletal muscle function in the hands and forearms. Their arm strength was measured by the Performance of the Upper Limb (PUL) test.
The test assesses patient’s ability to perform the arm tasks associated with daily living. arm strength as well as the ability to perform a variety of movements such as picking up coins, removing a container lid and lifting light weights. At that time, none of the patients who received standard medical therapy experienced improved arm strength and function.
Five of the 13 patients who received cells experienced temporary atrial fibrillation, an irregular and often rapid heart rate that can increase the risk of complications such as stroke and heart failure. Temporary atrial fibrillation is known to occur during cardiac catheterization in patients of this age range.
"We are now planning our Phase II trial, which will be a bit different," Victor said. "Instead of a one-time infusion during a cath lab procedure, the patients will receive the CDCs in an intravenous drip, and will receive multiple treatments spaced over several months."
Pending FDA approval, Victor said, the Phase II trial could begin in early-2018.
Source-Eurekalert
 MEDINDIA
MEDINDIA

 Email
Email






