A combination of drugs lumacaftor and ivacaftor were found to be safe and effective for children of ages 2-5 years in the treatment of cystic fibrosis.
‘Children suffering from cystic fibrosis responded well to the treatment of the drugs showing an improvement in growth by a significant increase in height, weight, and body mass index.’





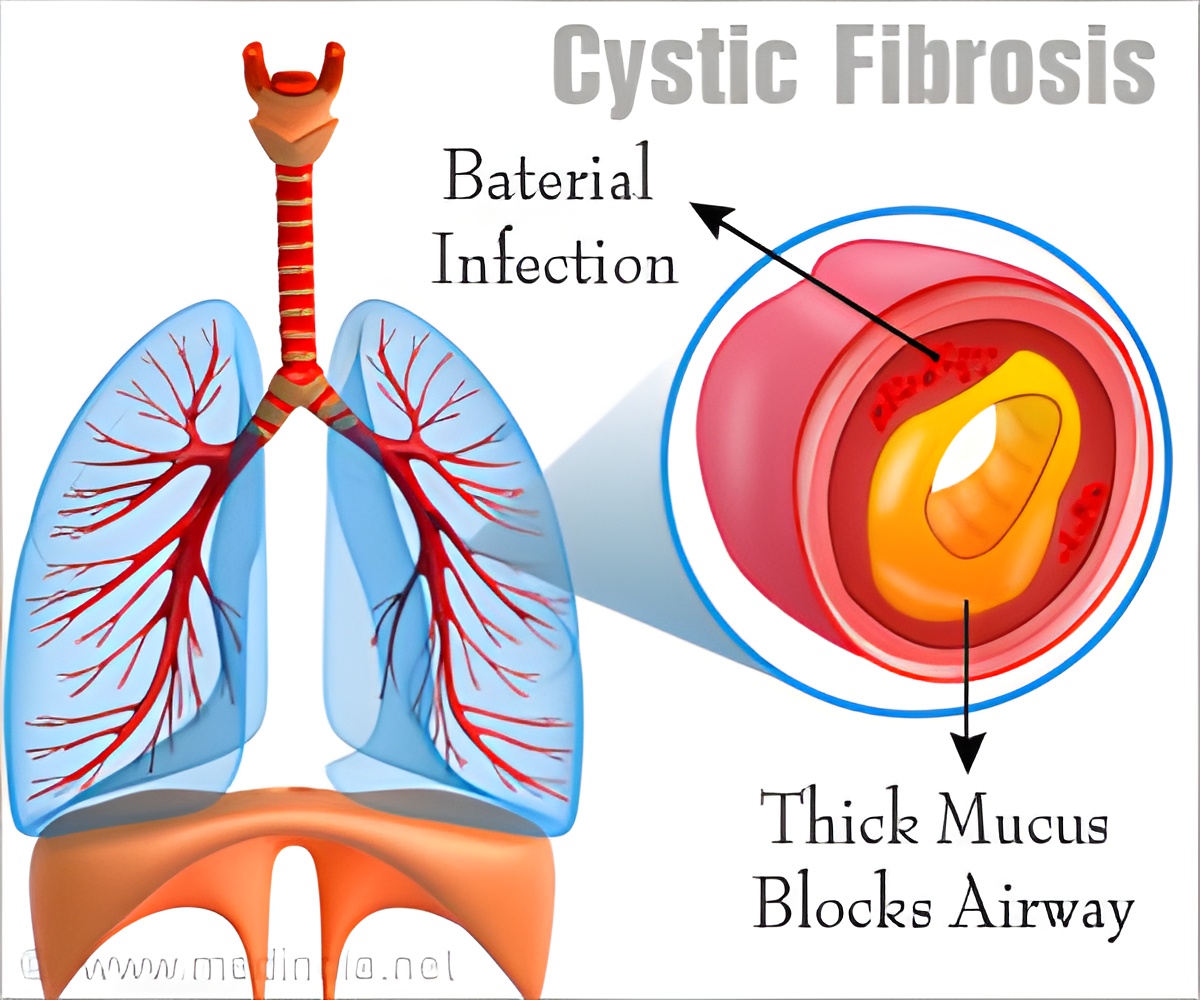
Cystic fibrosis is a progressive genetic disease that damages multiple organs, including the lungs and pancreas, with average predicted survival of 47 years. It is caused by mutations in the CFTR gene that lead to insufficient flow of salt and water in and out of cells. In the lungs, this creates buildup of thick, sticky mucus that can result in chronic lung infections and severe lung disease. Damage to the pancreas occurs even before birth, which interferes with nutrition absorption and growth. "Our study also showed that treatment with lumacaftor/ivacaftor can improve growth for children with cystic fibrosis," says Dr. McColley. "We saw significant increases in weight, height and body mass index over six months of treatment."
In addition to efficacy, the study also confirmed that lumacaftor/ivacaftor is well tolerated in the younger population. No new safety concerns were identified.
Dr. McColley is now leading an open label Phase 3 trial of this drug combination for children 1-2 years of age.
Advertisement