Keratitis, or inflammation of the cornea can cause serious complications or even permanent damage to the vision if left untreated.
TOP INSIGHT
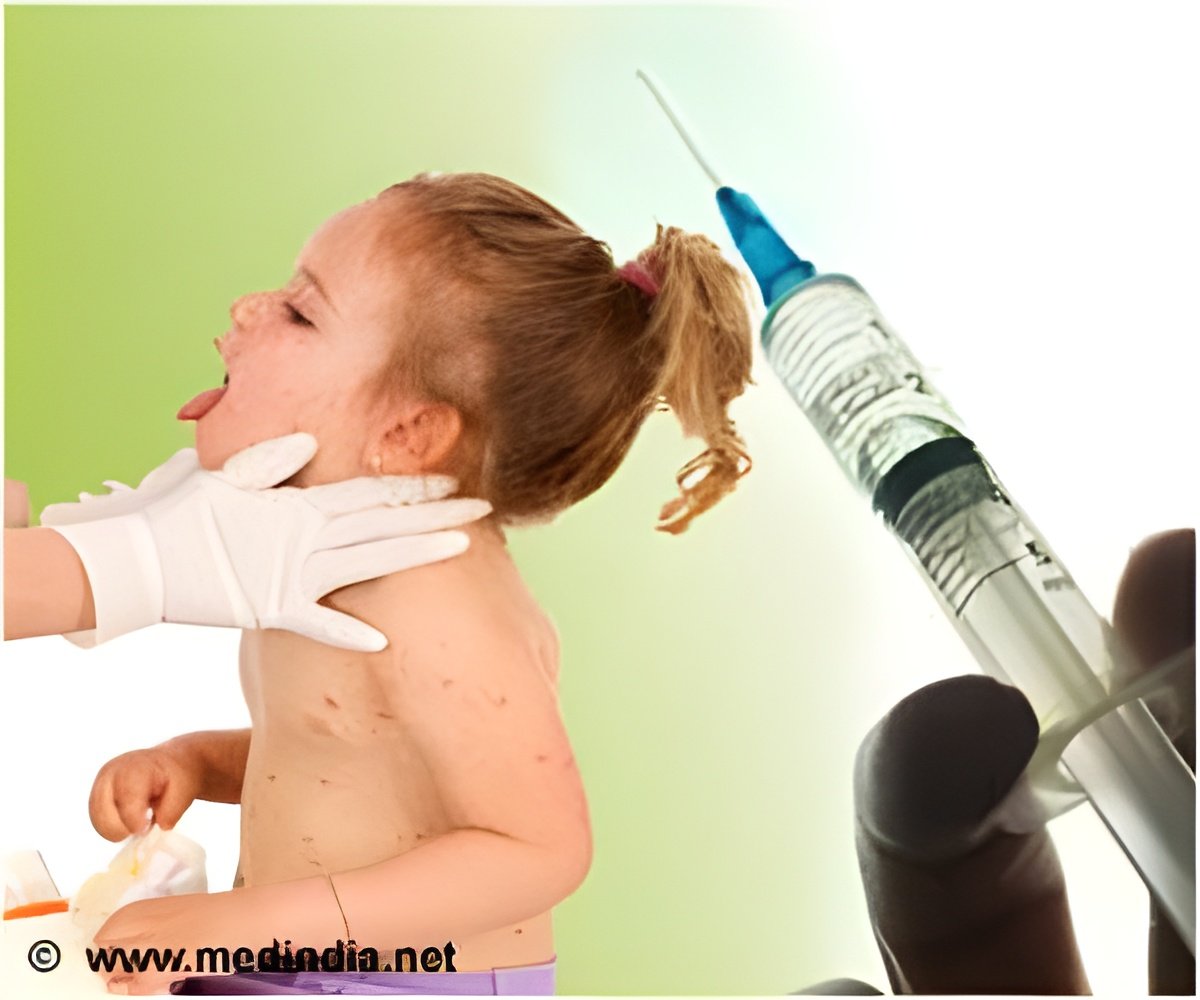
Chicken pox vaccine or varicella zoster virus vaccine can cause a serious side effect called as keratitis (corneal inflammation).
"Keratitis, or inflammation of the clear layer on the front of the eye, is a vision issue that can cause serious complications or even permanent damage to your vision if left untreated," said Frederick Fraunfelder, chair of the department of ophthalmology at University of Missouri School of Medicine in the US.
"By studying case reports from national and international registries, we found at least 20 cases of keratitis occurred in children and adults within a month of administration of the chickenpox and shingles vaccine," Fraunfelder said.
While this is a rare occurrence, it is important for physicians to know when giving the vaccine to individuals who have a history of the condition because it could be reactivated by the vaccine," Fraunfelder noted.
Fraunfelder is the director of the National Registry of Drug-induced Ocular Side Effects, an international effort to gather information on adverse ocular events associated with drugs, chemicals or herbs.
A review of the database and previously published reports found 20 cases of keratitis with a close relationship to administration of the vaccine. For adults, symptoms of keratitis developed within 24 days of vaccination. For pediatric patients, symptoms of inflammation developed within 14 days.
Source-IANS
 MEDINDIA
MEDINDIA


 Email
Email