American pharmaceutical giant Bristol-Myers Squibb has announced the immediate suspension of the development of its experimental hepatitis C drug.
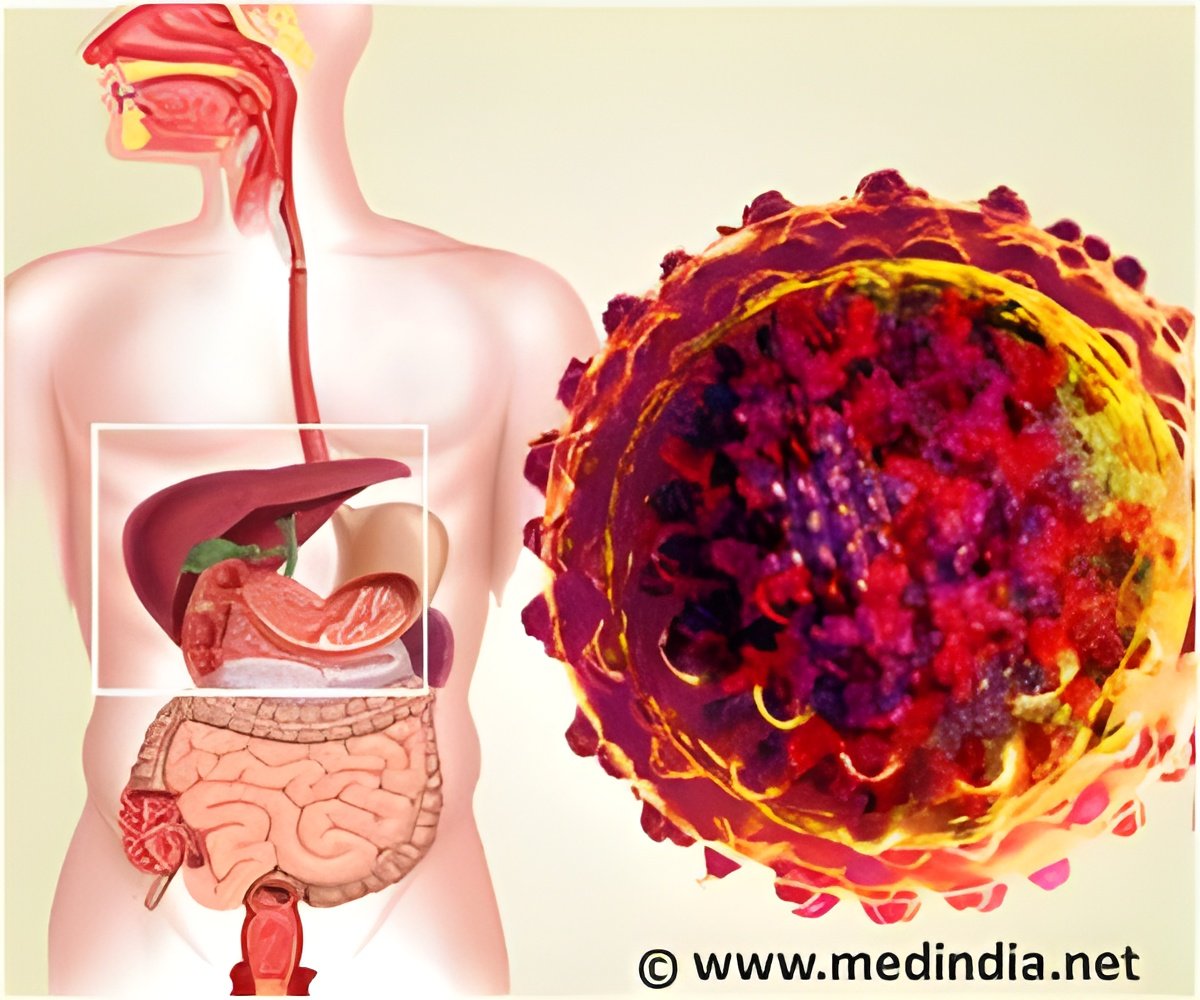
The trial was halted on August 1 following the death of a patient due to heart failure while a follow up on other patients led to the hospitalization of eight more. BMS-986094 belongs to a new class of drugs known as nucleotide polymerase inhibitors.
Bristol added that it is working closely with the US Food and Drug Administration to follow up on the remaining patients with its chief scientific officer, Elliott Sigal stating, “We will also work expeditiously to share the results of our further investigations more broadly in the medical and scientific community.”
Source-Medindia
 MEDINDIA
MEDINDIA


 Email
Email