A biomarker that can be used to accurately characterize the properties and function of mesenchymal stem cells (MSCs) has been identified by researchers.

The finding, published in the journal Cell Stem Cell on June 19, significantly advances the field of MSC biology, and if the same biomarker identified in CRI's studies with mice works in humans, the outlook for clinical trials that use MSCs will be improved by the ability to better identify and characterize the relevant cells.
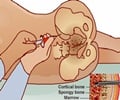
"There has been an increasing amount of clinical interest in MSCs, but advances have been slow because researchers to date have been unable to identify MSCs and study their normal physiological function in the body," said Dr. Sean Morrison, Director of the Children's Research Institute, Professor of Pediatrics at UT Southwestern Medical Center, and a Howard Hughes Medical Institute Investigator. "We found that a protein known as leptin receptor can serve as a biomarker to accurately identify MSCs in adult bone marrow in vivo, and that those MSCs are the primary source of new bone formation and bone repair after injury."
In the course of their investigation, the CRI researchers found that leptin receptor-positive MSCs are also the main source of factors that promote the maintenance of blood-forming stem cells in the bone marrow.
"Unfortunately, many clinical trials that are testing potential therapies using MSCs have been hampered by the use of poorly characterized and impure collections of cultured cells," said Dr. Morrison, senior author of the study and holder of the Mary McDermott Cook Chair in Pediatric Genetics at UT Southwestern. "If this finding is duplicated in our studies with human MSCs, then it will improve the characterization of MSCs that are used clinically and could increase the probability of success for well-designed clinical trials using MSCs."
Source-Eurekalert
 MEDINDIA
MEDINDIA


 Email
Email