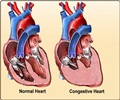
While both devices use the same technology, Consulta has both Medtronic’s exclusive OptiVol Fluid Status Monitoring, as well as Complete Capture Management™, which monitors and adjusts to patient needs automatically and can positively impact device longevity and reduce in-office testing.
OptiVol measures intrathoracic impedance in heart failure patients through monitoring the level of resistance to low electrical pulses, which indicates the patient’s fluid levels.
Complete Capture Management automatically adjusts the pace of the device to changing patient physiologic needs. It also helps clinicians to get rid of certain manual checks.
With the launching of these sophisticated devices, heart health care becomes more accessible and manageable.
Advertisement