- Delivery system with PowerTrac (TM) technology to provide superior deliverability through tight lesions.
- Environmentally friendly packaging, a reduced box size that has a smaller footprint on congested shelves and improved product labeling for fast readability to improve efficiency in the cath lab.
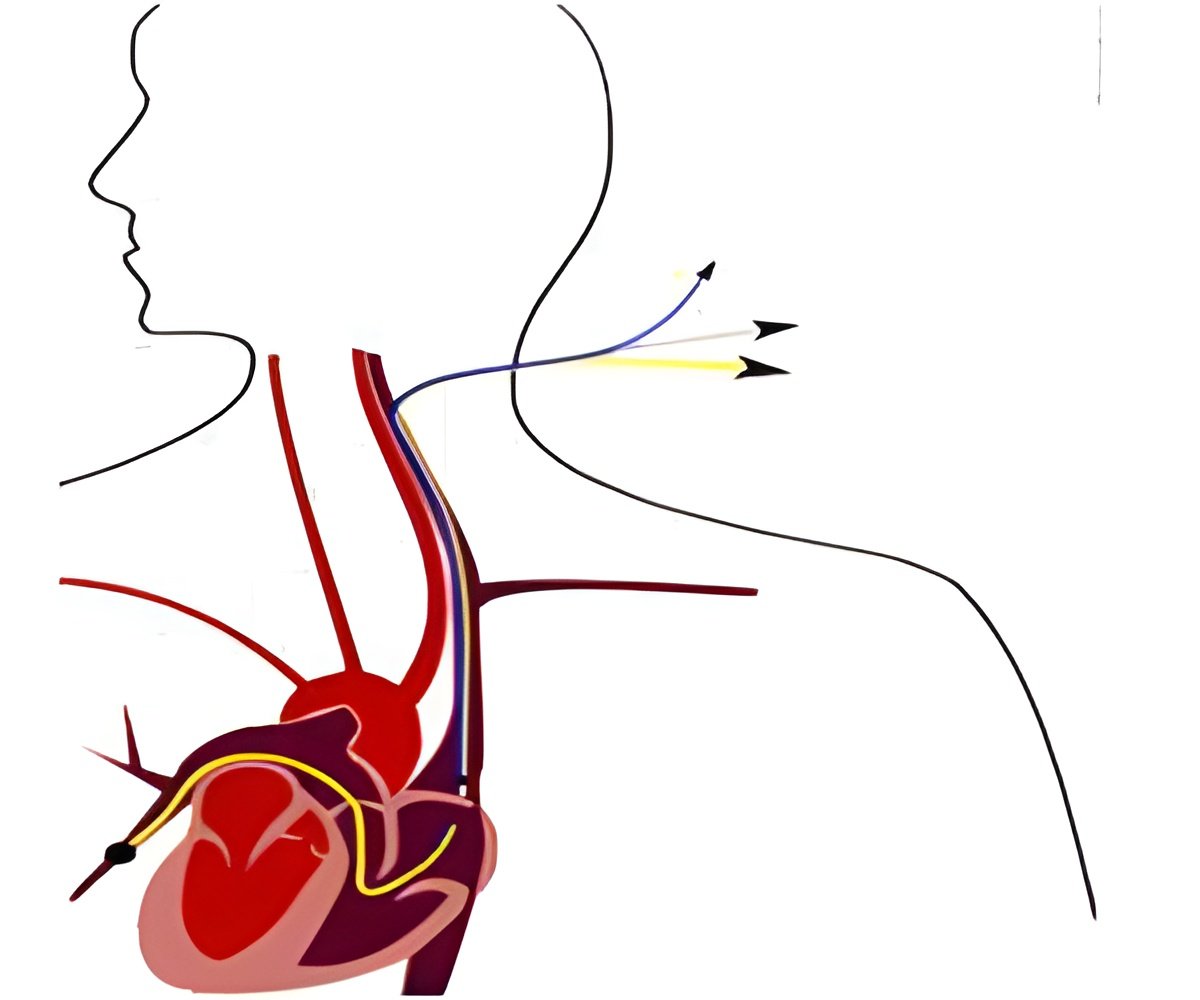
- Significantly improved insertion and retraction force to enhance navigation to lesion sites when using the Kissing Balloon Technique, a method for treating bifurcated lesions.
- Ultra-slim balloon material, a tapered proprietary inner shaft design and an optimized mini-wrap to reduce the wall thickness of the balloon and contribute to the extremely low crossing profile.
Source-Medindia


![Pulmonary Arterial Hypertension [PAH] - Symptoms & Signs - Causes - Diagnosis - Treatment Pulmonary Arterial Hypertension [PAH] - Symptoms & Signs - Causes - Diagnosis - Treatment](https://www.medindia.net/images/common/patientinfo/120_100/pulmonary-arterial-hypertension-pah.jpg)



