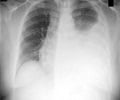
Delamanid is a novel tuberculosis treatment option to treat drug-resistant strains of Mycobacterium tuberculosis.
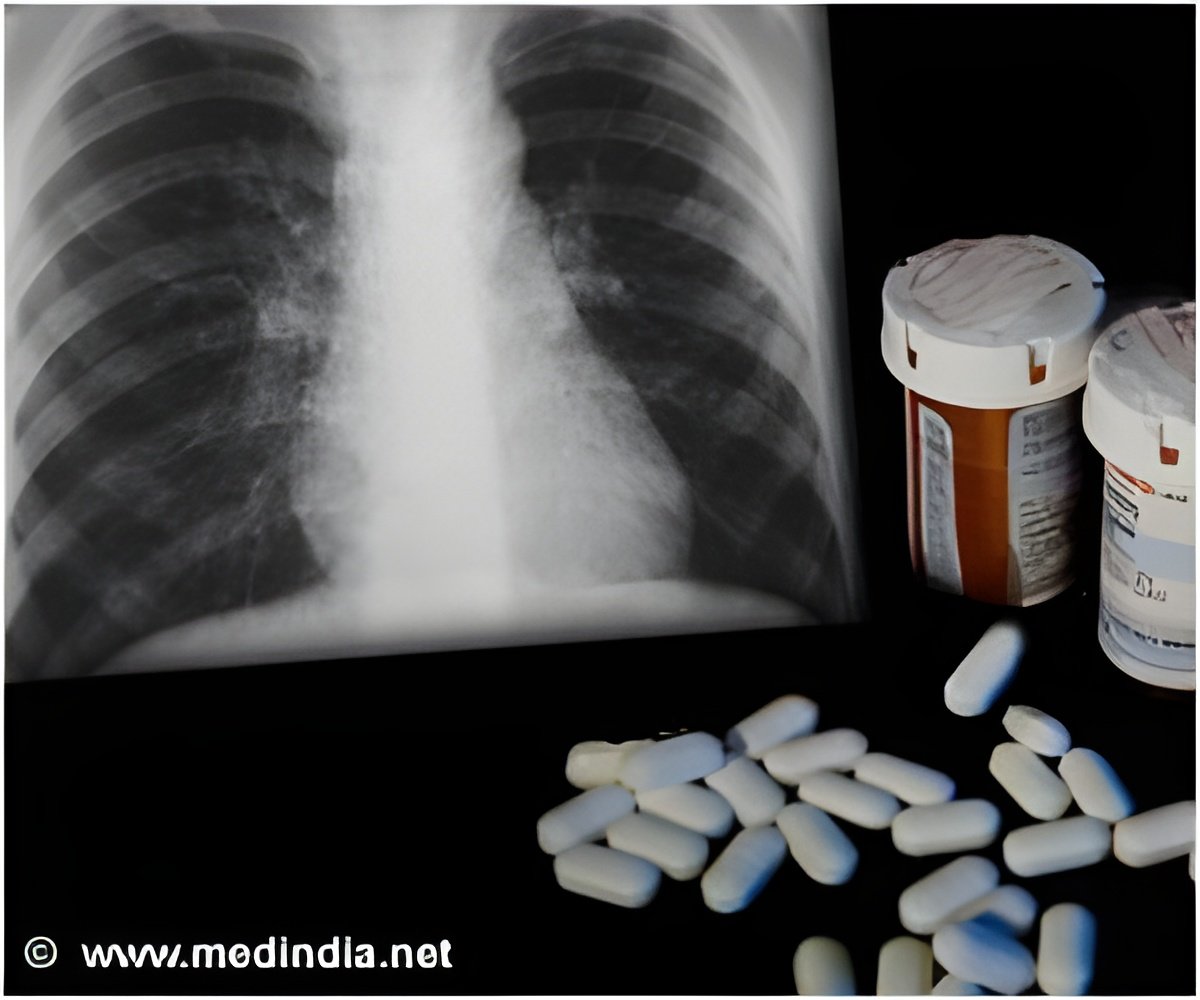
Delamanid (OPC-67683) is a drug from the nitro-dihydro-imidazooxazole family of mycolic acid synthesis inhibitors, which are pre-clinically proven to be effective against drug-responsive TB and MDR-TB. A 2-week study revealed that 200 to 300 mg of daily delamanid was as efficacious as rifampin in terms of antibacterial action.
The current clinical trial, across multiple countries, involved 481 patients aged 18 to 64 years and with sputum culture–positive MDR-TB and chest radiography consistent with TB. For 2 months, the patients received 100mg two-times-a-day delamanid (161 patients), 200 mg two-times-a-day (160 patients), or placebo (160 patients). Patients were also treated with a 4 to 5 drug combination therapy as per WHO recommendation for MDR-TB treatment. Sputum cultures were evaluated every week and sputum-culture conversion was said to occur when five or more sequential cultures were negative for M. tuberculosis. The cultures were considered positive based on the presence of colonies with specific morphological features.
Sputum-culture conversion was observed in 45.4% and 41.9% of 100mg and 200mg delamanid treated patients respectively as well as in 29.6% of placebo group patients. Adverse events were mild to moderate and evenly distributed among the groups.
Two-month treatment with delamanid promoted sputum-culture conversion in MDR-TB patients. Thus, delamanid may be the answer to the growing problem of multi-drug resistant tuberculosis.
Reference: Delamanid for Multidrug-Resistant Pulmonary Tuberculosis; Maria Tarcela et al; N Engl J Med 2012; 366:2151-2160 June 7, 2012
 MEDINDIA
MEDINDIA


 Email
Email