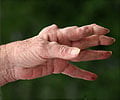
IL-17 induces production of anti-microbial peptides and pro-inflammatory cytokines that in turn may help sustain immune responses in the skin.4 With similar pathways impacting skin and joint diseases, data suggest that cytokine-targeting strategies aimed at blocking signalling through the IL-17 receptor may be a beneficial new strategy in the treatment of PsA.
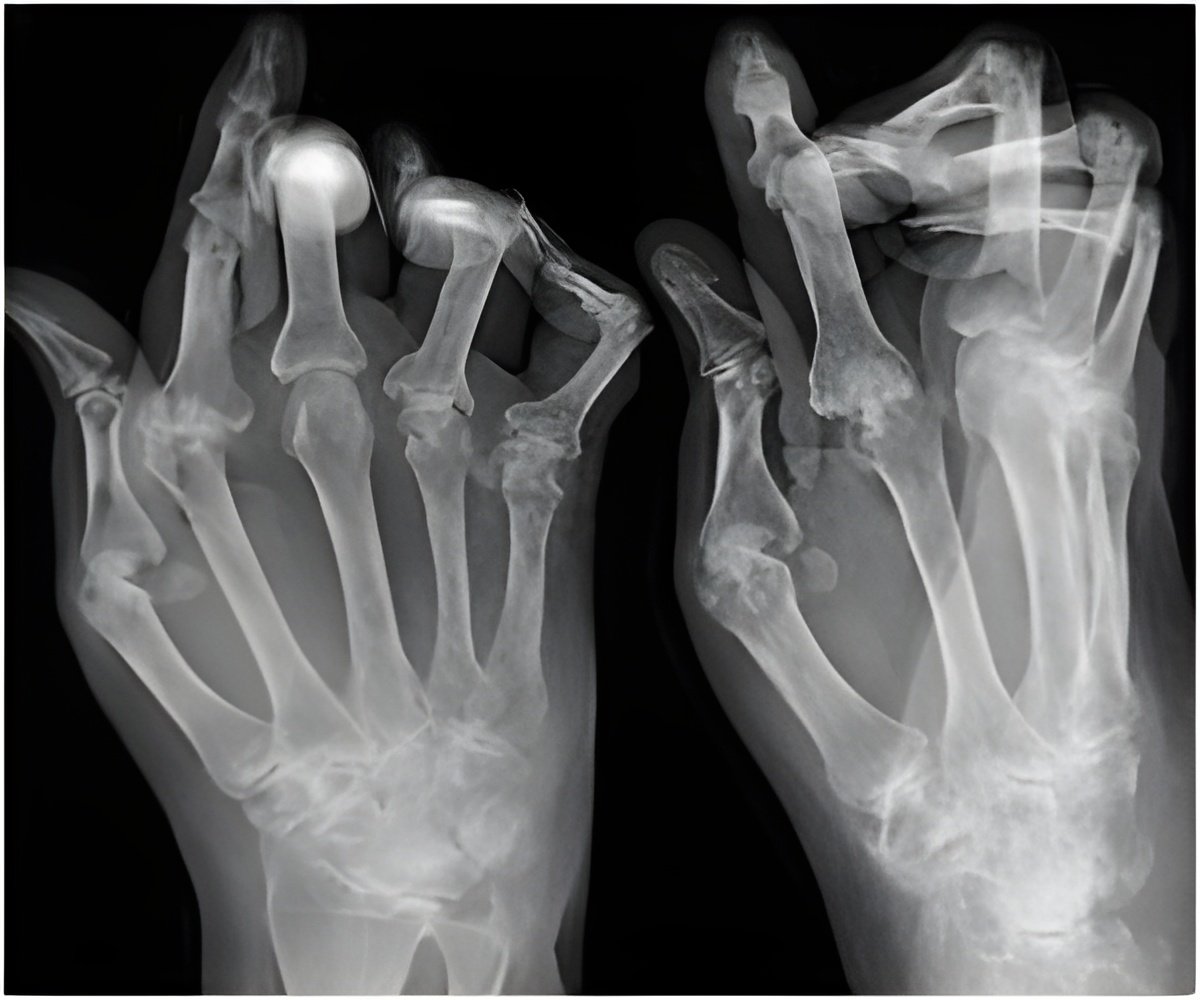
Lead author of the study Dr Mease, Swedish Medical Center and University of Washington, Seattle, US commented "PsA is a progressive disease associated with a number of co-morbidities, disability and disfigurement. There is a need for therapies to better manage patient outcomes, and prevent long-term bone loss and permanent joint damage, especially in those patients for whom anti-TNF therapy is not effective or tolerated." Dr Mease continued, "these significant patient responses support continued evaluation of brodalumab for the treatment of PsA and clearly show that cytokine-targeting strategies aimed at blocking signalling through the IL-17 receptor may represent an important new treatment strategy."
The study involving 168 patients with at least a 6 month history of PsA demonstrated that 37% and 39% of subjects in the 140- and 280-mg brodalumab groups respectively, achieved the primary endpoint of ACR20* response rates at week 12 compared with 18% of subjects in the placebo group (p <0.05).
A further analysis determined the ACR20 response rates in biologic-naïve subjects were 36% in the 140 mg group, 37% in the 280 mg group, and 20% in the placebo group; comparable with the respective rates of 37%, 42%, and 16%, in subjects with prior biologic exposure. There were consistent improvements in secondary endpoints including ACR50†, DAS 28 and components of the ACR including CRP.‡
Advertisement