The drug tiotropium (marketed as the Spiriva brand), can be delivered safely and effectively to people with chronic obstructive pulmonary disease (COPD) in both mist and traditional dry powder inhalers.
The study comparing the two drug-delivery systems was a randomized, double-blind, prospective trial conducted at 1,200 sites in 50 countries with more than 17,000 COPD patients. Results of the study are described in an article in the New England Journal of Medicine on Sept. 8, 2013, and are scheduled to be presented the same day at the European Respiratory Society meeting in Barcelona, Spain, by Robert Wise, M.D., professor of medicine at the Johns Hopkins University School of Medicine.
"This was a much-awaited study, because previous, smaller studies had raised the question of whether there was an increased risk of death with the newer tiotropium device, says Wise, who is the lead author of the study. "We needed a very large trial to answer the question."
The researchers randomly assigned participants to one of three groups. Two different doses of the drug were studied among those who used the new Respimat inhaler.
"Our study found that the safety profile of tiotropium delivered with the Respimat system, in either a dose of 5 micrograms or 2.5 micrograms, was similar to the traditional dose of the drug that is in wide use today delivered with the HandiHaler. In other words, there was no significant difference in the incidence of major cardiovascular events or risk of death among the three groups studied," Wise says.
Wise says the researchers also found that the risk of a first COPD exacerbation, defined as worsening of two or more major respiratory symptoms for at least three days, was no different when comparing the two devices or the different doses of the drug.
Death from any cause during the follow-up period occurred in 7.7 percent of patients in the lower-dose Respimat group, 7.4 percent of those in the higher-dose Respimat group and 7.7 percent among those patients using the HandiHaler.
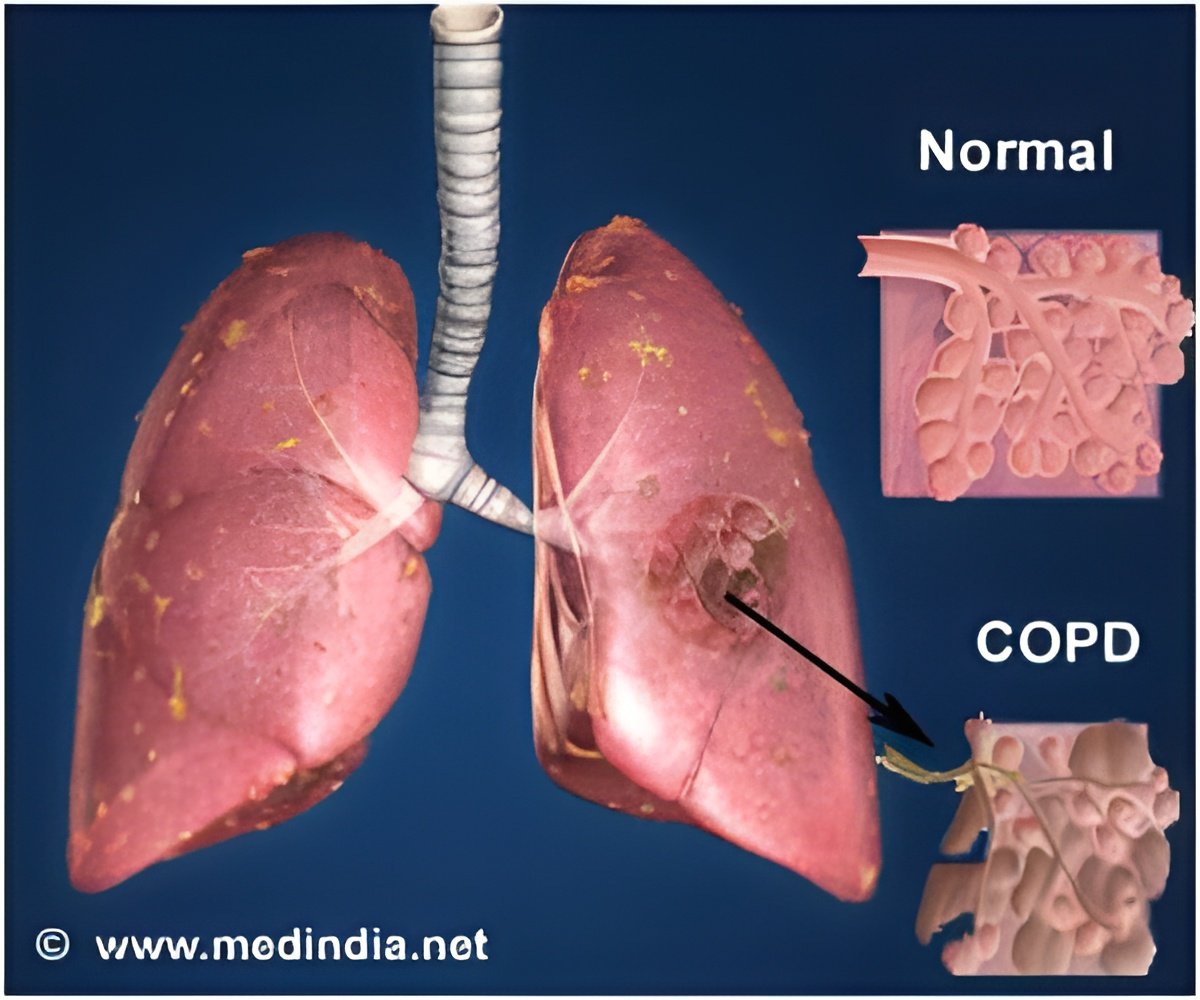
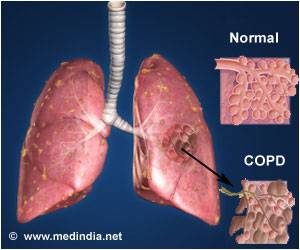
There is no cure for COPD, but inhaled medications can delay exacerbations of the disease and improve quality of life.
The study was funded by Boehringer Ingelheim, the pharmaceutical company that makes tiotropium and the two different delivery systems. Wise receives consulting fees from Boehringer Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline, Merck, Mylan, Novartis,
Pfizer, Sunovion, Pulmonx, Spiration, InterMune, Grifols and AstraZeneca. He receives grant support from Boehringer Ingelheim, GlaxoSmithKline, Pearl Therapeutics and Forest
Laboratories.
Other study authors include researchers from the University of Texas Health Science Center and South Texas Veterans Health Care system, Boehringer Ingelheim Pharmaceuticals, Odense University Hospital in Denmark, Service de Pneumologie Hospital Cochin, Assistance Publique-Hopitaux de Paris, University Paris Descartes, Sorbonne Paris City, and Institute of Ageing and Chronic Disease, University of Liverpool, United Kingdom.
JOHNS HOPKINS MEDICINE
Johns Hopkins Medicine (JHM), headquartered in Baltimore, Maryland, is a $6.7 billion integrated global health enterprise and one of the leading health care systems in the United States. JHM unites physicians and scientists of the Johns Hopkins University School of Medicine with the organizations, health professionals and facilities of The Johns Hopkins Hospital and Health System. JHM’s mission is to improve the health of the community and the world by setting the standard of excellence in medical education, research and clinical care. Diverse and inclusive, JHM educates medical students, scientists, health care professionals and the public; conducts biomedical research; and provides patient-centered medicine to prevent, diagnose and treat human illness. JHM operates six academic and community hospitals, four suburban health care and surgery centers, more than 38 primary health care outpatient sites and other businesses that care for national and international patients and activities. The Johns Hopkins Hospital, opened in 1889, was ranked number one in the nation for 21 years by U.S. News & World Report.
Source-Newswise
 MEDINDIA
MEDINDIA
 Email
Email