Stanford University School of Medicine scientists have generated beating heart cells that carry the same genetic mutation by using skin cells
In a study to be published online Feb. 9 in Nature, the investigators also report their identification of a promising drug to reverse the heart malfunction — for which there are currently no decent treatments — after using these newly created heart cells to check the effects of a plethora of compounds.
The new approach involved converting skin cells to heart cells in a dish by reprogramming them to an embryonic-stem-cell-like state, so that the cells are capable of "differentiating" into a multitude of cell types. The scientists then chemically coaxed these induced pluripotent stem cells to become heart cells. The iPS-cell approach represents a big advance because no good alternative methods for studying human heart malfunction at the cellular level now exist.
"This may be the first time this noninvasive 'disease-in-a-dish' technique has been used successfully to screen for drugs in heart disorders," said Ricardo Dolmetsch, PhD, associate professor of neurobiology and senior author of the study. The study's first author is Masayuki Yazawa, PhD, a postdoctoral researcher in Dolmetsch's lab.
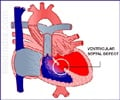
The human heart is a pump made of muscle and consisting of four compartments, or chambers: left and right ventricles and two corresponding atria. These chambers must contract in a coordinated sequence to ensure orderly blood flow. That coordination is mediated by electrical signals from cardiac nodes, which are to the heart's chambers what sparkplugs are to a car engine's pistons. In the aggregate, signals among heart cells generate electromagnetic waveforms that can be visualized on an electrocardiogram.
Nearly a dozen genetic mutations identified in humans are known to cause disruptions in this signaling pattern, resulting in a condition called long QT syndrome. (The name reflects an elongated interval between two portions of the waveform typically observed in an electrocardiogram.) People with LQTS suffer from arrhythmias, or irregular heartbeats, and are vulnerable to ventricular fibrillation, an often fatal state in which heart cells contract chaotically.
Advertisement
For their Nature study, Dolmetsch and his colleagues turned to patients with Timothy syndrome, one genetic mutation known to cause LQTS. Patients with Timothy syndrome are highly susceptible to ventricular fibrillation and often die at an early age. Another hallmark feature of Timothy syndrome is autism, which is the primary focus of Dolmetsch's research.
Advertisement
Exactly why calcium-channel malfunction in Timothy syndrome patients causes cardiac arrhythmia has not been known. One big reason research into both the causes of and treatments for LQTS in general has lagged is that it's hard to study heart cells, said Dolmetsch. "It would be dangerous and unethical to extract heart cells from a living person with or without cardiac disease," he said. In theory, the gene defect tied to Timothy syndrome could be reproduced in a laboratory mouse, whose heart could then be studied. But in practice, this is a non-starter. While a healthy person's resting heart rate is about 60 beats per minute, a mouse's heart thumps at a rate of 500 times a minute, making the organ useless for analyzing timing deficits that afflict human hearts.
The study marks an exciting use of iPS cells, a relatively new technology that was first introduced in 2006. Dolmetsch and his associates reprogrammed skin cells from two Timothy syndrome patients and five normal individuals first into iPS cells, then into cardiomyocytes. Three distinct varieties of cardiomyocytes — atrial, ventricular and nodal cells — were generated in this way from both diseased as well as normal subjects. The three cell subtypes spontaneously clumped into miniature heart-like organs resembling a one-chambered heart.
It was apparent that, in contrast to the average 60 beats per minute of the "miniature hearts" derived from normal subjects' skin cells, those of Timothy syndrome patients beat at about a 30-per-minute rate and showed substantial irregularities. The investigators dissected these tiny organs into their constituent cells and showed that each was composed of atrial, ventricular and nodal cells.
Significantly, Dolmetsch's group found that in the Timothy syndrome-derived ventricular cells, but not atrial or nodal cells, the calcium channels encoded by the mutant gene opened normally to allow calcium flow but stayed open longer than those of normal cells. With special dyes that mirror calcium concentrations, Dolmetsch and his team were able to visually inspect calcium flow in heart cells prepared from Timothy syndrome patients' skin.
"We found that their ventricular cells, although not their atrial or nodal cells, had impaired calcium flow" compared with like cells from normal subjects, said Dolmetsch.
The investigators examined the response of these irregular-beating cells to different drugs that have been reported to affect heartbeat rhythms. When they added one of these drugs — roscovitine, currently in clinical trials for an unrelated indication — to the cell-culture medium at the right dose, the deficient calcium flow was restored, and so was the regular heartbeat.
Dolmetsch cautioned that at this point roscovitine should not be considered an adequate treatment for LQTS — it hasn't been tested for this purpose in living animals, let alone humans, and may have pronounced side effects. Still, he said, it's a promising compound for further drug development. Stanford's Office of Technology Licensing has applied for U.S. patents related to the discovery, and Dolmetsch is starting a new company that intends to license those patents once they're granted.
Source-Eurekalert