The dengue vaccine, Dengvaxia, manufactured by the French pharmaceutical giant Sanofi has received approval from regulators in three countries.
TOP INSIGHT
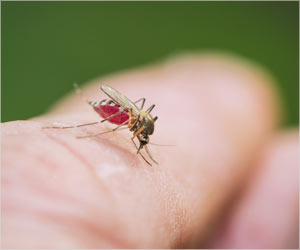
Dengue is now endemic in more than 100 countries due to globalization, urbanization, climate change and air travel that helps it to move into more temperate zones.
Mexico became the first country to allow the vaccine on December 9, and the Philippines followed suit last week.
Sanofi has requested regulatory approval in 20 countries across Asia and Latin America.
The vaccine is a potential "blockbuster" drug for the company, which estimates it could generate more than $1 billion a year in revenue.
The Brazilian government said regulators must still set a price per dose, a process that takes about three months on average.
Scientists have long been stumped by dengue, which has four separate strains.
Dengue infects as many as 400 million people per year, and the deadliest form kills 22,000 per year, says the World Health Organization.
It was once considered a disease of the tropics, endemic in only nine countries.
But globalization, urbanization, climate change and air travel are helping it to move into more temperate zones. It is now endemic in more than 100 countries.
The WHO says cases have risen 30-fold over the last 50 years, with more than half the world's population potentially at risk.
The 20 countries where Sanofi Pasteur hopes for regulatory approval have a total population of two billion people -- 200 million of them in Brazil.
Source-AFP
 MEDINDIA
MEDINDIA

 Email
Email