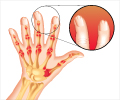
New drug compound named filgotinib decreased joint swelling and tenderness among rheumatoid arthritis patients within 12 weeks.

‘Patients with treatment-resistant rheumatoid arthritis could benefit from the novel drug which substantially reduced symptoms, improved daily physical functioning and remission rates.’
Read More..




"For patients who haven't done well on other therapies, these findings are cause for optimism, enthusiasm and hope," said Mark Genovese, MD, professor of immunology and rheumatology and principal investigator of the study. Read More..
Stanford Health Care offers services to rheumatoid arthritis patients through its immunology and rheumatology clinic. As clinical chief, Genovese spends 3 half-days per week in the clinic, where he regularly sees and treats rheumatoid arthritis patients.
He is the paper's lead author. The senior author is Tsutomu Takeuchi, MD, PhD, professor of rheumatology and clinical immunology at Keio University School of Medicine in Tokyo, Japan.
Rheumatoid arthritis is a progressive, systemic autoimmune disease affecting at least 1 in every 100 people worldwide. For reasons that aren't understood, 3 of every 4 people with the disorder are women. While its most visible hallmarks are pain, stiffness, inflammation and eventual deterioration of joints, patients also are at heightened risk for cardiovascular disease and other inflammatory complications.
In clinical trials, about 70% of rheumatoid arthritis patients have appeared to benefit initially from small-molecule therapies in a pill form, such as methotrexate, said Genovese, who is the James W. Raitt M.D. Professor.
Advertisement
Selective JAK-1 inhibitor
Advertisement
The trial was conducted in 114 centers in 15 countries, mostly in North America and Europe. The 449 participants averaged 56 years of age, and about 80% of them were female. They were randomized to 1 of 3 study arms, in which they received daily doses of either 200 milligrams of filgotinib, 100 milligrams of filgotinib or a placebo for 24 weeks. All participants had moderately to severely active rheumatoid arthritis despite treatment with one or more biologic therapies.
The primary goal of the study was to observe whether there was an improvement at 12 weeks into the trial of at least 20% on a measure of joint swelling and tenderness called the ACR20 that was established by the American College of Rheumatology. An important secondary outcome was a score indicating low disease activity in 28 predetermined joints on a test called the DAS28-CRP.
Compared with the placebo group, a significantly greater proportion of participants on both the high- and low-dose filgotinib regimens achieved the primary endpoint: a 20% improvement in symptoms as measured by the ACR20. Sixty-six percent of participants on 200 milligrams of filgotinib, and 57.5% of those on 100 milligrams, fulfilled this criterion, versus only 31.1% of those on placebo.
Of equal or even greater importance, Genovese said, was the participants' improvement on the DAS28-CRP at both 12 and 24 weeks. By 12 weeks, 40.8% of those on the 200 milligram dose of filgotinib and 37.3% of those on 100 milligrams had reached the status of low disease activity as measured by the DAS28-CRP, as opposed to only 15.5% of those on the placebo regimen. These outcomes continued or improved over the course of the trial. By 24 weeks, 48.3% of the high-dose filgotinib recipients and 37.9% of those on the low dose had reached low-disease-activity status.
By week 12 of the trial, 22.4% of high-dose and 25.5% of low-dose filgotinib recipients, but only 8.1% of placebo recipients, had DAS28-CRP scores indicating outright remission. By week 24, high-dose recipients had a remission rate of 30.6%; low-dose recipients, 26.1%; and placebo recipients, 12.2%.
Improvement seen early on
The drug's benefits to participants became apparent soon after the trial's onset. "We could see improvements as early as two weeks into the trial," Genovese said.
Also telling was a substantial difference among the study arms in how many participants completed the 24-week trial. Of the 148 participants in the placebo arm, 51 dropped out before completion. Only 20 of the 148 high-dose recipients and 34 of the 153 low-dose recipients dropped out.
Investigators' early concerns about increased susceptibility to infections, or the re-emergence of active forms of prior infections, such as tuberculosis or shingles, were assuaged by the relative smattering of such adverse events, compared with placebo.
Notably, patients for whom at least three different biologic therapies provided insufficient relief did as well in this trial as those who'd derived insufficient relief from just one biologic therapy, Genovese said.
"We found that those high levels of response were independent of how many drugs you'd failed, and independent of which drugs you'd failed," he said.
Overall response rates to filgotinib appear to surpass those of the other commercially available JAK inhibitors at doses approved for use in the United States, he said.
"This novel drug works exceptionally well in patients who've already failed traditional therapies for rheumatoid arthritis," he said.
Source-Eurekalert