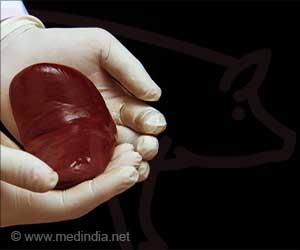
Pig-organ transplants: what three human recipients have taught scientists
Go to source).
‘Within 48 hours post-transplant, human immune cells infiltrated pig organs, taking control of signaling and influencing efforts to prevent irreversible cellular damage in xenotransplants. #xenotransplantation #immuneresponse’
Tweet it Now
Advertisement
Single-Cell Analysis Reveals Cellular Changes During Xenotransplantation
Two new analyses, published online on May 17 in Nature Medicine and on May 21 in Med, have unveiled changes at the single-cell level in the organs and recipients’ bodies before, during, and immediately after the xenotransplantation surgeries in the decedants. Teams of scientists collaborated with the surgeons, collecting blood and tissue samples to examine alterations in tens of thousands of cells.Led by researchers at NYU Grossman School of Medicine and the Broad Institute of MIT and Harvard, the Med paper tracked the genetic and cellular activity in the two pig kidneys transplanted into humans, and compared them against pig kidney samples that had not been translated. To do so, the research team used several techniques, including single-cell RNA sequencing, which determined the order (sequence) of the molecular letters making up the pig and human genes active in various cell types during the procedures.
Advertisement
Immune Response and Rejection Mechanisms in Pig Kidney Xenotransplantation
The study showed that the transplanted pig kidneys, while not rejected outright by the recipients’ bodies (no immediate kidney failure), caused a strong reaction in human peripheral blood mononuclear cells (PBMCs). This set of immune cells can attack transplanted (foreign) organs much like they attack foreign invaders (e.g. viruses). While immediate rejection was not seen, in part due to treatment with medications that suppressed it, the new study found evidence of subtler reactions that could cause xenotransplants to fail over time.Specifically, the pig kidneys were seen to trigger “antibody-mediated rejection” at the molecular level. As the body develops immune proteins called antibodies specific to a transplanted organ, they recruit natural killer cells, macrophages, and T cells that can injure it. The team also saw an uptick in pig kidneys of tissue repair mechanisms, where certain cells multiply as part of the growth involved in healing. Normal cells that transform into cancer cells also grow aggressively, so the mechanism bears watching.
“We have detailed the cellular mechanisms that dictate how human immune cells react to a xenotransplant in the short term,” said Jef Boeke, PhD, a co-senior author on both studies, and director of the Institute for System Genetics at NYU Grossman School of Medicine. “These results give us new insights into how we might further engineer pig organs for transplant, or tailor immunosuppression treatments to improve tolerance of a foreign organ.”
By tracking the interplay between the kidneys and human system several times each day, the researchers found that pig kidney immune cells drove reactions right after the transplant, but that human immune cells infiltrated the pig organs by 48 hours to dominate signaling. Measuring the degree to which pig immune cells trigger the first wave of immune attack on xenotransplants will shape efforts to prevent irreversible cellular damage to them, say the study authors.
Advertisement
Transplanted Hearts: Multiomics Analysis and Immune Response in Pig Heart Xenotransplantation
The other new paper, published in Nature Medicine, featured a “multiomics” analysis of pig hearts and surrounding human cells in decedents. This included analyzes every six hours after transplant of gene activity (transcriptomics), as well of proteins (proteomics), lipids, and metabolites (intermediates in biological pathways) present in cells.Rapid, massive increases in the number of certain cell types were also seen in decedents receiving pig hearts. In one of the decedents (D1) but not the other, activated T cell and natural killer (NK) cell populations within the PBMC group increased from about one percent 30 hours post-transplant to more than 20 percent of the entire PBMC population by 66 hours after the procedure. This dramatic immune reaction to the organ, a complication called perioperative cardiac xenograft dysfunction (PCXD), came with a damaging inrush of immune cells (inflammation), and misplaced healing attempts (tissue remodeling) that thicken tissue and can hinder function.
The worse outcomes experienced by the one decedent may be partly because this heart was smaller than anticipated for the recipient’s size, and required an extra procedure to compensate for it, the researchers said. These factors may have cut off blood flow and the oxygen supply to the heart for longer, which is known to cause ischemia reperfusion injury when the supply is restored. The research team observed that PCXD-related immune reactions to the pig organ got worse in the presence of this recipient’s reperfusion injury.
Reference:
- Pig-organ transplants: what three human recipients have taught scientists - (https://www.nature.com/articles/d41586-024-01453-2)
Source-Eurekalert