A novel therapeutic approach has been identified by scientists for the most frequent genetic cause of ALS.
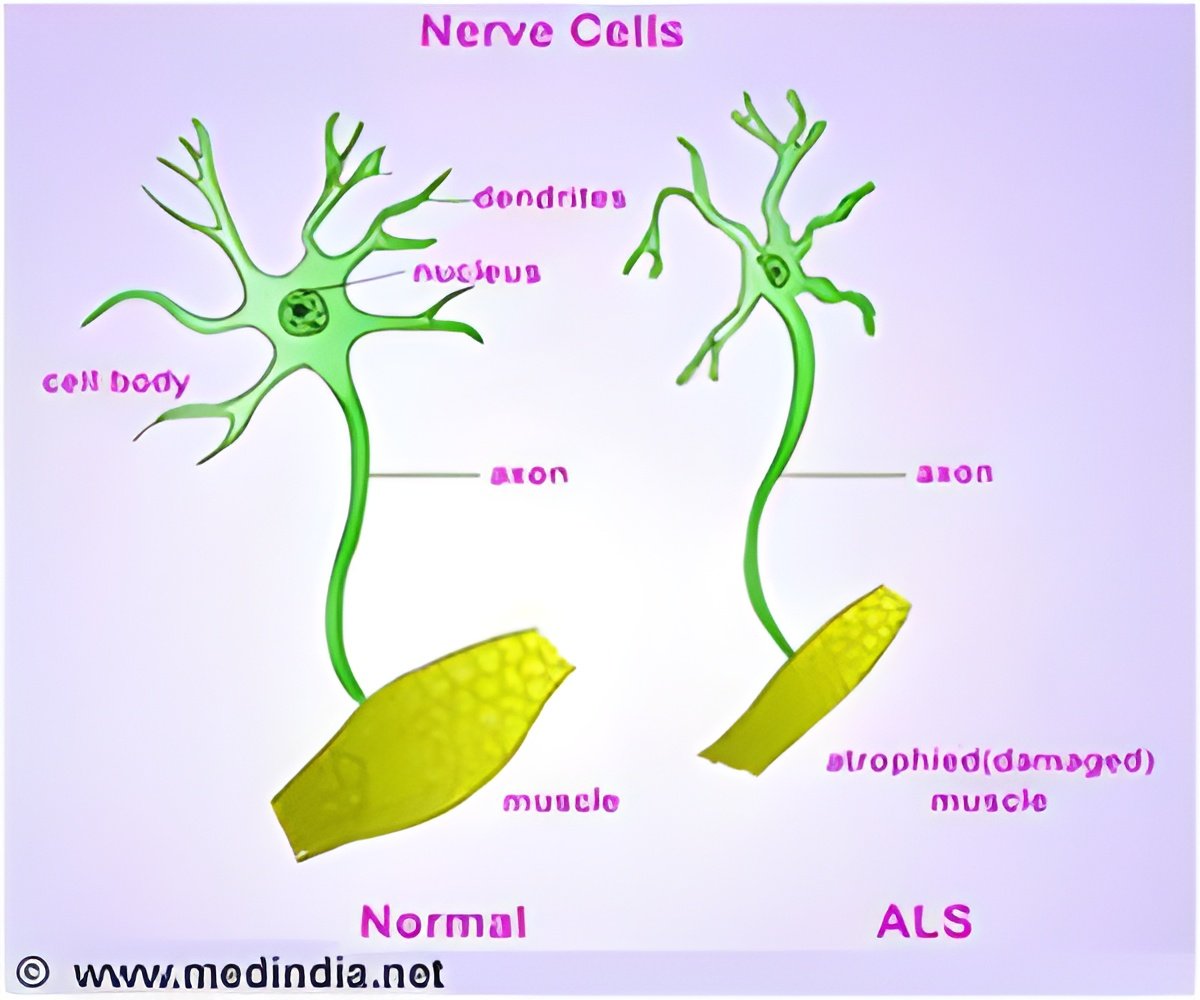
The study establishes using segments of genetic material called antisense oligonucleotides - ASOs - to block the buildup and selectively degrade the toxic RNA that contributes to the most common form of ALS, without affecting the normal RNA produced from the same gene.
The new approach may also have the potential to treat frontotemporal degeneration or frontotemporal dementia (FTD), a brain disorder characterized by changes in behavior and personality, language and motor skills that also causes degeneration of regions of the brain.
First author Clotilde Lagier-Tourenne, MD, PhD, UC San Diego Department of Neurosciences and Ludwig Institute for Cancer Research, said that remarkably they found two distinct sets of RNA foci, one containing RNAs transcribed in the sense direction and the other containing anti-sense RNAs.
The researchers also discovered a signature of changes in expression of other genes that accompanies expression of the repeat-containing RNAs.
Since they found that reducing the level of expression of the C9orf72 gene in a normal adult nervous system did not produce this signature of changes, the evidence demonstrated a toxicity of the repeat-containing RNAs that could be relieved by reducing the levels of those toxic RNAs.
She said that importantly, they showed that they could remove the toxic RNA without affecting the normal RNA that encodes the C9orf72 protein.
The study has been published online in the journal PNAS.
Source-ANI
 MEDINDIA
MEDINDIA


 Email
Email





