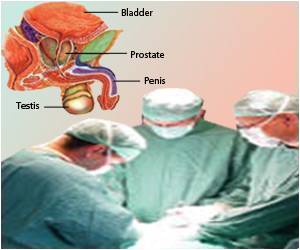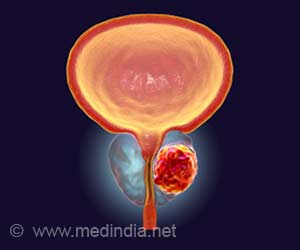
‘Immunotherapy stimulates body’s own immune system to fight cancer. Immunotherapy medications used in different genitourinary cancer of kidney, bladder, and prostate include- nivolumab, ipilimumab, pembrolizumab, avelumab.’
Read More..Tweet it Now
Immunotherapy is used in combination with other therapies like surgery, targeted therapy, chemotherapy, or radiation. Immunotherapy acts by stimulating body’s own immune system to fight cancer. It is the second choice of therapy in case conventional treatments cause intolerable side effects or if cancer does not respond to or returns after traditional treatment.Read More..
Robert Alter, M.D., co-chief of Urologic Oncology at the John Theurer Cancer Center said, “Immunotherapy is usually a tolerable therapy that provides patients with an excellent quality of life. We certainly want to add years to each patient’s life, but we also want to add life to those years.”
Immunotherapy does not cause symptoms like nausea and hair loss commonly seen with chemotherapy hence, it is well-tolerated by patients. Patients should be carefully monitored for signs of toxicity like an overactive immune response leading to inflammation in the body.
Dr Alter said, “Our clinical care providers, including our nurse navigators, nurse practitioners and medical assistants, are tuned in to each of our patients and are constantly watching for side effects due to immunotherapy. Cancer care is a team effort.”
Targeting Immunotherapy
Advertisement
Dr Alter said, “ Next-generation sequencing allows us to choose treatments that can selectively target a patient’s tumor with limited toxicity, achieving a better response and enabling the patient to have a better quality of life. It’s not only about the cancer; it’s also about the patient who has the cancer.”
Advertisement
In kidney cancer, immunotherapy is effective at decreasing the size of the tumors. This helps the patients to live longer and their quality of life is improved.
In the treatment of kidney cancer, Interleukin-2 (IL-2) and interferon-alpha use cytokines which is a type of immune-stimulating chemical. Cytokines are a type of signaling molecules that help regulate immunity. Because of an increased risk of serious side effects, this therapy is used as a second choice of treatment.
Immune checkpoints are a part of the immune system that prevents healthy cell’s destruction due to a strong immune response. Proteins on tumor cells bind to these checkpoints which prevent the immune system from destroying the cancer cells.
Checkpoint inhibitors that target specific proteins and restore the ability of the immune cells to fight cancer are the newer medications. These drugs can be used in combination or alone to target specific proteins on the tumor.
In a study called CheckMate 214, 11 percent of patients with stage IV kidney cancer who used two checkpoint inhibitors- nivolumab combined with ipilimumab were able to achieve complete remission.
For the treatment of advanced kidney cancer, immunotherapy drug pembrolizumab combined with targeted drug axitinib is the first choice. According to a study, 59 percent of patients showed decrease in size of the tumor.
To prevent the spread and return of cancer, these immunotherapy medications can also be used as maintenance therapy.
Dr Alter said, “Patients with advanced kidney cancer are receiving the benefit of multiple types of therapies that can be used in combination and offer increased convenience, tolerability and effectiveness. Now, in addition to discussing convenience, tolerability and effectiveness, we are also able to discuss curability and survival, which we weren’t able to do 15 years ago.”
Role of Immunotherapy in Bladder Cancer
In patients who are not able to tolerate chemotherapy or those in whom chemotherapy is ineffective, immunotherapy can be an option. Bladder cancer can be treated by using immune checkpoint inhibitors given as intravenous (IV) infusion every 2 to 3 weeks, similar to kidney cancer.
According to the clinical trial JAVELIN Bladder 100, 50 percent improvement in the overall survival for patients was seen when they received the drug avelumab as maintenance immunotherapy.
Dr Alter said, “Having the ability to switch treatment mechanisms if one treatment isn’t working gives patients reassurance. We are taking science and moving cancer care forward.”
Role of Immunotherapy in Prostate Cancer
Hormone therapy called androgen suppression therapy suppresses the male hormone androgen so that it can no longer stimulate prostate cancer cells. Hormone therapy, along with immunotherapy and targeted therapies, are used to treat prostate cancer.
Hormone therapy can be given as an injection which shows its action in 4-6 weeks or it can also be given as a daily oral medication which shows its action in just one week.
Dr Alter said, “In addition to exploring new, effective treatment options, we are also looking at how we administer medications and the impact that may have on a patient’s quality of life.”
Advanced Clinical Trials
The John Theurer Cancer Center routinely participates in various leading-edge clinical trials. They provide patients who have genitourinary cancer access to some of the most promising therapies years before they receive FDA approval and become available to the public.
Dr. Alter shared a story about a patient suffering from cancer which continued to recur despite standard chemotherapy treatment surgery. Now the patient’s cancer has been in complete remission for four years after he participated in a Phase I clinical trial. One year before the medication received FDA approval, the patient finished treatment with the clinical trial drug.
Data from the clinical trial helps the oncologists to expand into new effective therapies that will improve patient’s quality of life.
Dr Alter said, “Our patients are like family, and I always encourage them to participate in clinical trials. These studies can give patients a running start on treatment before a drug receives FDA approval. When I do my charting at the end of the day, I often find myself smiling because my patients are doing so well. I feel like every day is going to be a rewarding day, and I am always excited about the next patient success story.”
Source-Medindia