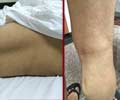
Belgian researchers seem to have identified the mechanism behind Charcot-Marie-Tooth (CMT) neuropathy, a hereditary disease.
Now VIB researchers have developed a mouse model for. They also found a potential therapy for this incurable disease. The treatment not only halted the damage to the nerves and the atrophy of the muscles, it even succeeded in reversing the symptoms. The research was conducted under supervision of Wim Robberecht en Ludo Van Den Bosch from VIB-K.U.Leuven, in collaboration with the team of Vincent Timmerman at VIB-University of Antwerp. The study was published in Nature Medicine.
Earlier work by VIB researchers showed that some CMT patients have mutations in HSPB1, a gene coding for the 27 kDa small heat shock protein B1, a protein that plays a role in many stress-related molecular processes in the body. Until now, it was unclear how mutations in HSPB1 could lead to degeneration of the nerve bundles and to muscular weakness.
The core of the study by Constantin van Outryve d’Ydewalle consists of the construction of a mouse model for CMT. The researchers expressed the mutated human HSPB1 gene in mouse neurons. The mouse model develops motor symptoms, muscle atrophy and weakness, foot deformities and steppage gait, all very similar to symptoms observed in affected individuals. Furthermore, the mice develop sensory problems that also occur in CMT patients. Pathological examination of the nerves of the CMT mice shows that the contact between the nerve endings and muscles is disturbed.
The CMT mice provide the unique possibility to isolate and culture affected nerve cells, making it possible to investigate what exactly goes wrong in the sick nerves. It was discovered that the transport of mitochondria (the cellular power plants) within the axons is severely disturbed in the neurons from symptomatic CMT mice, most likely because the tracks along which the mitochondria are transported (microtubules) are damaged. This could lead to a chronic lack of sufficient mitochondria and other transported cargoes at the nerve endings, causing the nerves to degenerate.
These new insights also open possibilities for treatment, because the mitochondrial transport in nerve fibers is known to be affected by tubulin deacetylation, a posttranslational modification of the building blocks of microtubules catalyzed by histone deacetylase 6 (HDAC6). Inhibitors of HDAC6 do not only reverse the axonal transport deficits in vitro, treatment of the CMT mice with HDAC6 inhibitors also halts the damage to the nerves and even succeeds in reversing the symptoms, most likely by muscle reinnervation. The most specific therapeutic molecule used in this study (Tubastatin A) was made by Alan Kozikowski from the University of Illinois at Chicago (USA).
Reduced axonal transport in neurons is also observed in other neurodegenerative or neurological diseases, opening the door for further investigations into the effects of this new therapeutic strategy in other diseases. Further scientific research is crucial to solve this issue.
Source-Medindia
 MEDINDIA
MEDINDIA

 Email
Email