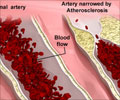
A team of researchers in Japan have managed to conduct a viable bypass in pigs by using ostrich blood vessels.
Conventional substitutes -- taken from dead human donors, animals or made of synthetic fibres or resins -- need to be at least double that in order to prevent problems with clotting.
Chief researcher Tetsuji Yamaoka said the arteries, which carry blood to the ostrich's head, are processed and lined with clot-preventing molecules on a nano scale.
"Ostriches are good as they provide a stable supply of narrow and long vessels," said Yamaoka, who heads the Biomedical Engineering Department of the National Cerebral and Cardiovascular Centre in Suita, western Japan.
Researchers at Yamaoka's laboratory used the new vessel in femoral artery bypass operations in five miniature pigs, bridging large arteries in their right and left thighs.
They confirmed the new vessel allowed blood to flow smoothly without the use of clot-prevention agents, Yamaoka said this week, calling it the world's first success in small-diametre, long bypass operations in animals.
"But vessels must be narrow and long to be used in humans," he said, adding at least 10 centimetres would be needed for human heart operations and 20 centimetres for legs.
Yamaoka's team aims to start clinical tests in three years.
Source-AFP
 MEDINDIA
MEDINDIA


 Email
Email