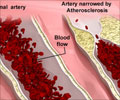
The treatment approach to heart disease could soon be changed thanks to new strategies involving vaccines and monoclonal antibodies to combat atherosclerosis.
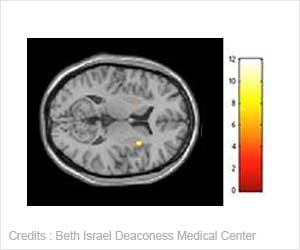
"People at high risk of MI are likely to be the first candidates for immune approaches. Such treatments, since they've totally different modes of action, could be used in addition to the current therapies," explained Nilsson, who is professor of Experimental Cardiovascular Research at Lund University, Sweden, and a key player in the development of immune treatments.
Speaking at the session "Atherosclerosis and the immune system: Expanding beyond inflammation"¹, Nilsson said that with phase 2a trials on recombinant antibodies currently ongoing in the US and Canada and results expected to be announced Quarter IV 2012, such treatments could soon become a clinical reality. "If all goes well, the first in class of these treatments could be licensed within four to five years," he added.
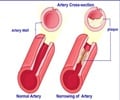
Undoubtedly there is an urgent need for new approaches to tackle CHD, a condition that kills two million people across Europe each year. Established therapies against atherosclerosis almost exclusively focus on risk factor modification – that is reduction of dyslipidaemia, hypertension and hyperglycaemia. "Existing treatments only reduce the risk of patients experiencing a CVD event by 40%. Although such results are encouraging, it shouldn't be forgotten that 60% of CVD events continue to occur," said Nilsson.
It was in the early 1990s that identification of antibodies against oxidised low density lipoproteins (LDL) in artery plaques, first gave rise to the concept that CVD might be an autoimmune disease where the immune system attacks oxidised LDL. To test this hypothesis Nilsson and colleagues conducted experiments immunizing rabbits with high blood cholesterol with their own oxidised LDL. "We had anticipated that immunization would result in the atherosclerosis becoming more aggressive, but to our initial disappointment found that immunization appeared to be activating protection against atherosclerosis. At the time this made no sense to us at all," he recalled.
The team subsequently discovered that through serendipitous use of an adjuvant (agent added to vaccines to increase the immune response) they had in fact stumbled upon a way to shift the T cells from pro-inflammatory Th1 responses towards protective Th2 and regulatory T cell responses. "This had the overall effect of dampening down inflammation and reducing the plaque severity," said Nilsson.
The resulting CVX-210 vaccine, currently in development by CardioVax, involves one of these three amino acid fragments (residues 3136 to 3155). CardioVax are currently awaiting FDA clearance to start phase 1 clinical trials with the vaccine, which will be given subcutaneously. Also in development is a second vaccine using the same amino acid sequence that has been formulated in a way that makes it possible to give intranasally.
Preclinical studies show that administration of the BI-204 monoclonal antibody, developed jointly by BioInvent and Genentech, reduced the formation of atherosclerotic plaques and plaques already present by 50%. In the phase I study, which took place in 80 healthy volunteers with elevated levels of LDL, BI-204 was found to be safe and well tolerated.
Now for the current phase 2a double blind GLACIER (Goal of oxidised Ldl and Activated maCrophage Inhibition by Exposure to a Recombinant antibody) study, BI-204 is being delivered intravenously to 144 patients with stable coronary artery disease in addition to standard care. The study, which is taking place at 20 centres in the US and Canada, has been designed to demonstrate reduction in inflammation in the carotid artery quantified by FDG-PET imaging (18Fluoro-2-deoxyglucose positron emission tomography) at weeks four and 12 following initiation of treatment. At the beginning of March, the companies announced that patient recruitment was completed.
Looking to the future, Nilsson said it was unlikely that either the monoclonal antibodies or vaccine would be given as "one off jabs" during childhood against CVD. "Both these treatments are far more like drugs – to be effective they'd need to be given long term. The antibody therapy in particularly is likely to be expensive so you could probably only afford to give it to high risk populations rather than everyone," he said.
Source-Eurekalert
 MEDINDIA
MEDINDIA

 Email
Email