Single infusion of hematopoietic stem and progenitor cells in a mouse model of Friedreich's ataxia measurably restored normal cellular functions, reports study.
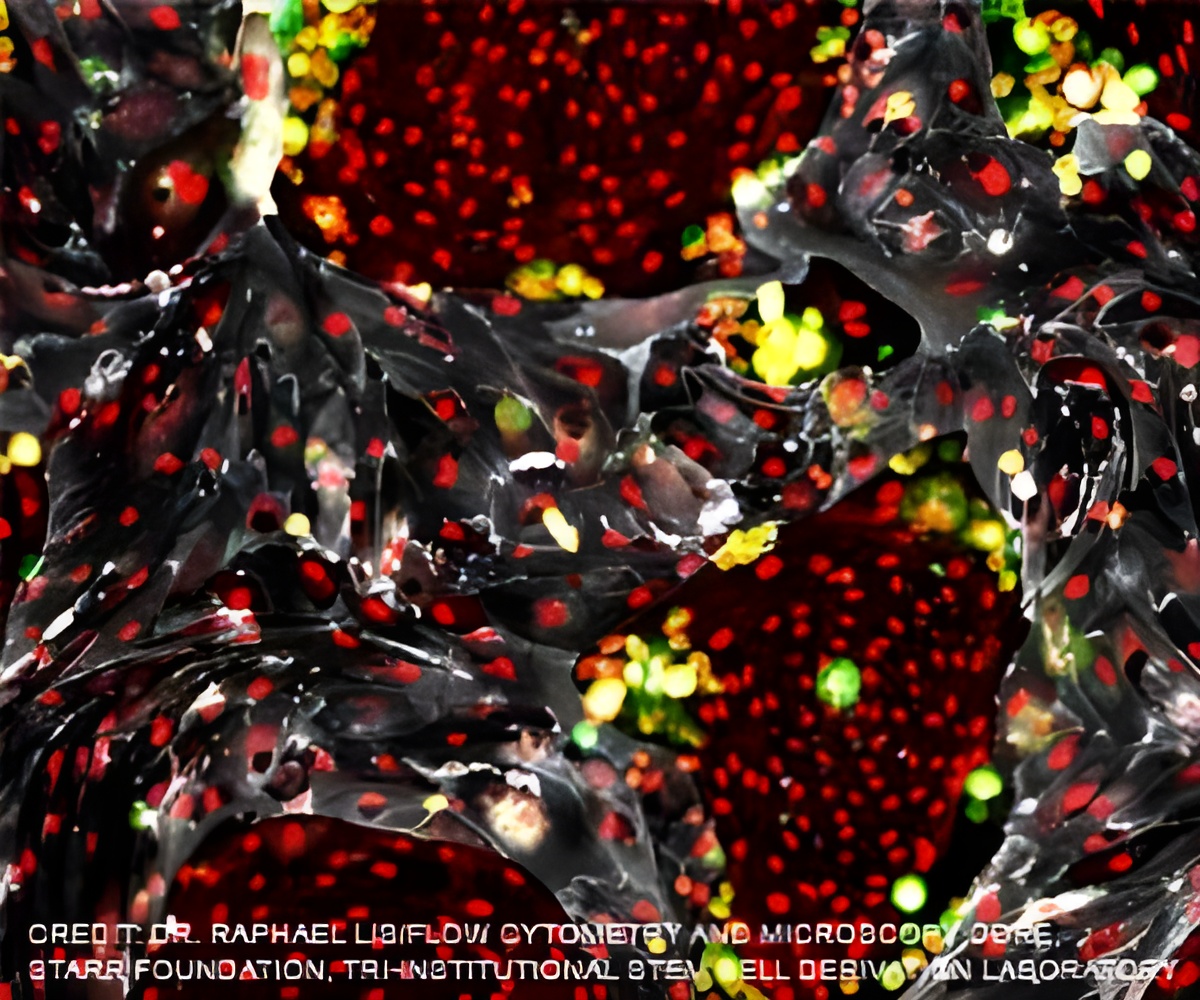
TOP INSIGHT
Transplanting wildtype HSPCs into an FA mouse model, appeared to transfer wildtype FXN into deficient neurons and muscle cells.
Human hematopoietic stem and progenitor cells (HSPCs), derived from bone marrow, have become a primary vehicle for efforts to replace or regenerate cells destroyed by a variety of diseases. Previous research by Cherqui and colleagues had shown that transplanting wildtype or normal mouse HSPCs resulted in long-term kidney, eye and thyroid preservation in a mouse model of cystinosis, another genetic disorder.
In this study, Cherqui's team transplanted wildtype HSPCs into an FA mouse model, reporting that the HSPCs engrafted and soon differentiated into macrophages in key regions of the mice's brain and spinal cord where they appeared to transfer wildtype FXN into deficient neurons and muscle cells.
"Transplantation of wildtype mouse HSPCs essentially rescued FA-impacted cells," said Cherqui, "Frataxin expression was restored. Mitochondrial function in the brains of the transgenic mice normalized, as did in the heart. There was also decreased skeletal muscle atrophy."
The scientists note that the mouse model is not perfect mirror of human FA. Disease progression is somewhat different and the precise pathology in mice is not fully known. However, Cherqui said the findings are encouraging and point toward a potential treatment for a disease that currently has none.
Source-Eurekalert
 MEDINDIA
MEDINDIA


 Email
Email