Omalizumab, the life saving asthma drug has been approved for the treatment of asthma by NICE in the United Kingdom.
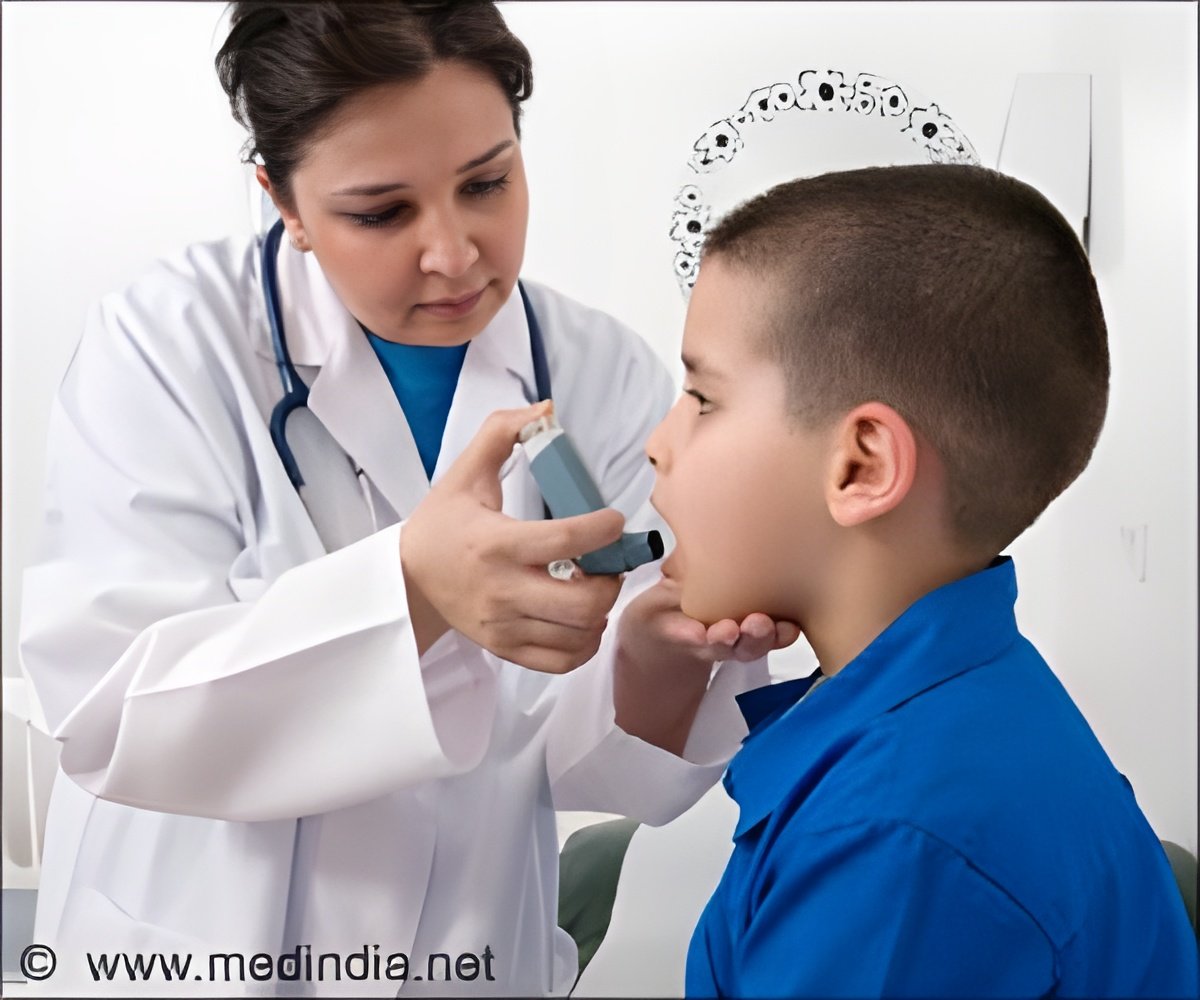
Newer medications like Omalizumab have helped to deal with cases of severe allergic asthma. The use of this medication has reduced the need and therefore helped to avoid the side effects of asthma. It is administered in the required dose as an injection every 2 to 4 weeks and acts by preventing severe asthma attacks, thereby reducing the need for hospitalization and decreases the chances of deaths due to asthma.
Omalizumab had been approved earlier in 2007 by the National Institute for Health and Care Excellence (NICE) in the United Kingdom for adults and children over the age of 12 years only after they had been admitted at least once for a severe asthma attack. However, it was later discontinued since NICE felt that it was less affordable and effective than it had thought.
Due to outcry from the patients as well as the medical community, as well as based on the results of various drug trials in asthma patients, Omalizumab has again been re-introduced; this time, children between the ages of 6 and 11 years are also eligible for the medication. Though the individuals do not require undergoing hospitalization to be eligible for the treatment, they have to undergo at least four courses of steroids before they can receive this medication.
This decision by NICE will hopefully benefit a large number of patients of severe asthma in the United Kingdom.
Source-Medindia
 MEDINDIA
MEDINDIA


 Email
Email