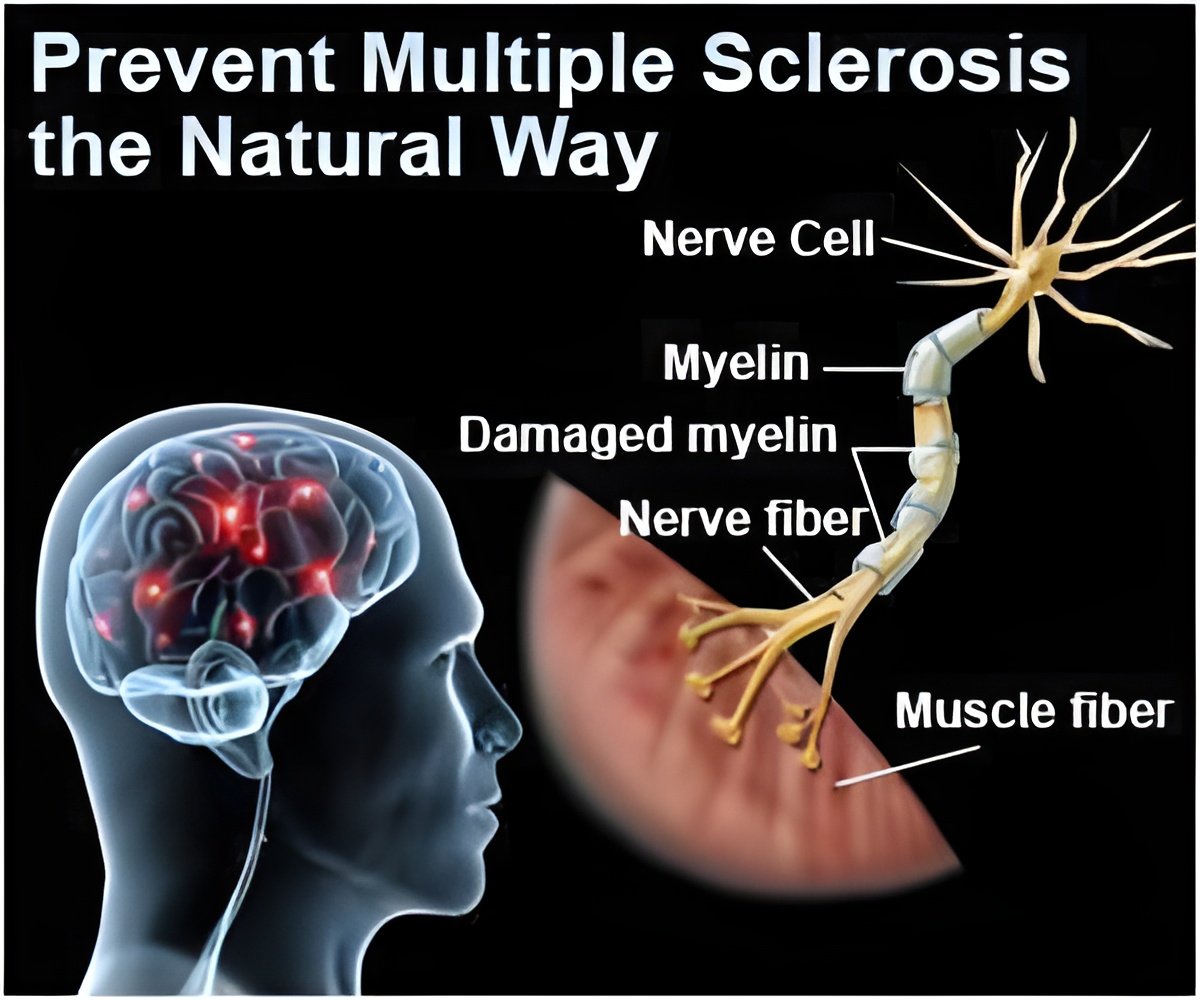
The 20 mg generic version of the drug could be labeled ‘substitutable’, meaning prescriptions for Copaxone could be automatically switched to the generic.
However, the companies are still engaged with the patent litigation. Sreejit Mohan, spokesman of Sandoz, said the company would not comment on commercial launch plans.
Sources with Momenta said the 20 mg generic version of the blockbuster drug could be labeled ‘substitutable’, meaning prescriptions for Copaxone could be automatically switched to the generic.
Teva’s U.S. sales of Copaxone totaled $3.1 billion in fiscal 2014. The company has been working to switch patients to a 40 mg formulation of the drug that still has patent protection.
Source-Medindia
 MEDINDIA
MEDINDIA


 Email
Email




