Study shows first-of-its-kind gene therapy can restore vision to people with an inherited retinal disorder.
TOP INSIGHT
Voretigene neparvovec, gene therapy, restored light sensitivity in patients with inherited blindness and enabled them to navigate in low light.
Approval could open the door for other gene therapies that could eventually treat the more than 225 genetic mutations known to cause blindness. It could be applied to retinitis pigmentosa, another inherited retinal disease caused by a defective gene. Or in the future, gene therapy could possibly provide key proteins needed to restore vision in more common diseases such as age-related macular degeneration.
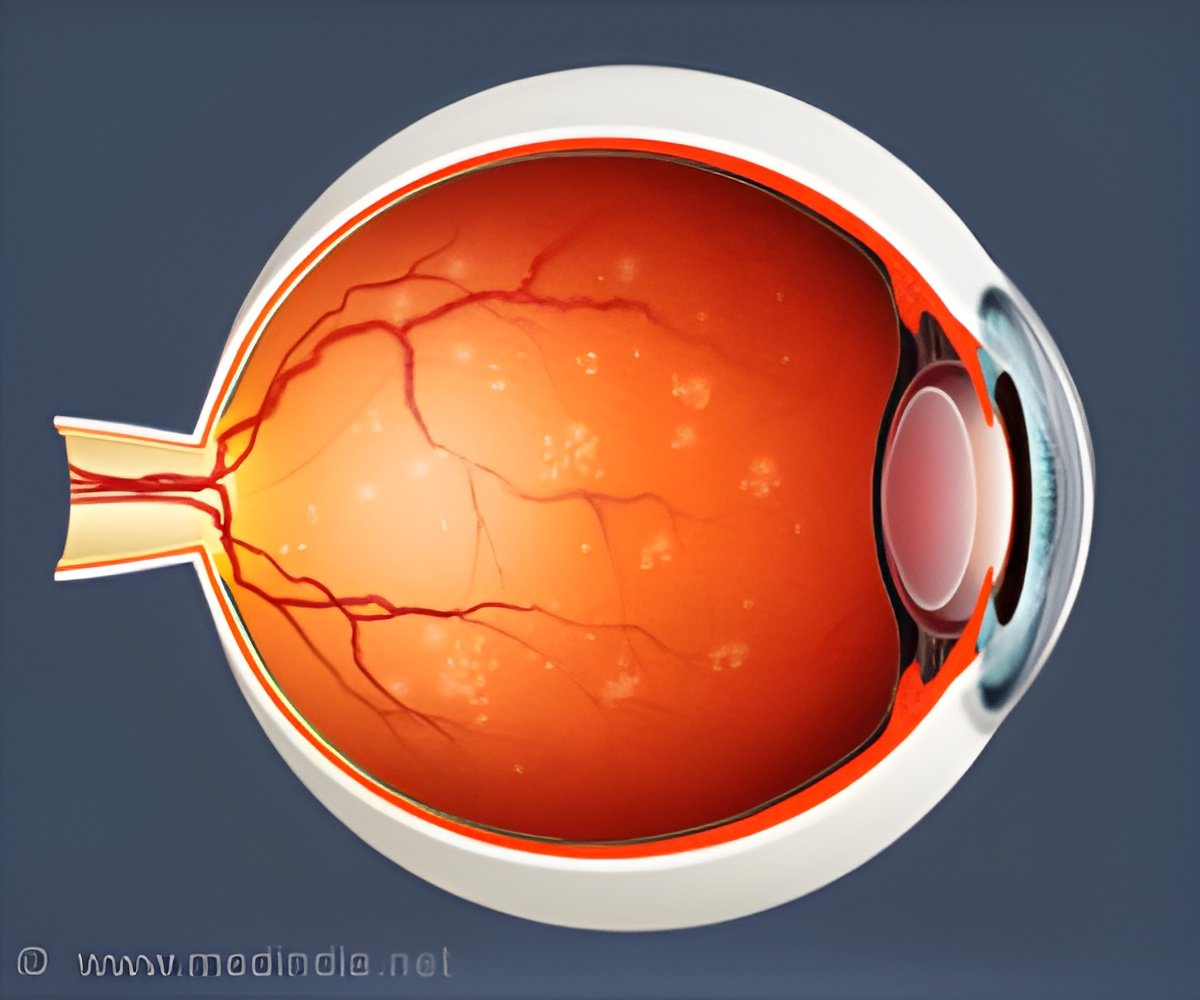
LCA is rare, affecting about 1 in 80,000 individuals. It can be caused by one or more of 19 different genes. The treatment, called voretigene neparvovec (Luxturna, Spark Therapeutics), involves a genetically modified version of a harmless virus. The virus is modified to carry a healthy version of the gene into the retina. Doctors inject billions of modified viruses into both of a patient’s eyes.
Treatment doesn’t restore normal vision. It does, however, allow patients to see shapes and light, allowing them to get around without a cane or a guide dog. It is unclear how long the treatment will last, but so far, most patients have maintained their vision for two years.
More than 200 patients with LCA have participated in gene therapy trials since 2007. However, no gene therapy has gotten this close to FDA approval for retinal disease or any other eye disease. In October, an advisory committee to the FDA unanimously endorsed the treatment. The FDA isn’t obligated to follow the recommendations of its advisory committees, but it usually does. The agency is expected to make its decision no later than January 2018.
Source-Eurekalert
 MEDINDIA
MEDINDIA


 Email
Email










