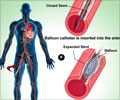
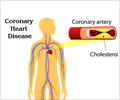
Food and Drug Administration, USA, gave its nod to the use of vorapaxar tablets under the name Zontivity to reduce the risk of heart attack, stroke and cardiovascular death.
It took some time for the drug to get the green signal as there were some concerns related to bleeding in patients who were put on the medicine during clinical trials.
“In patients who have had a heart attack or who have peripheral arterial disease, this drug will lower the risk of heart attack, stroke, and cardiovascular death,” said Ellis Unger, director of the Office of Drug Evaluation I in the FDA's Center for Drug Evaluation and Research.
According to the FDA, the medicine will carry a warning about the risk of intracranial haemorrhage (ICH). Thus, it is not approved for patients who have suffered a stroke. Zontivity blocks a receptor called protease-activated receptor-1 (PAR-1) and thus prevents clotting as it stops blood platelets from coagulating.
The approval came only after FDA advisory panel voted 10-1 in favour of the drug in January. As per Merck statistics, each year about 190,000 Americans face a second heart-related event.
Source-Medindia
 MEDINDIA
MEDINDIA


 Email
Email