The U.S. Food and Drug Administration (FDA) has approved a new drug called Cometriq to treat a rare form of thyroid cancer.
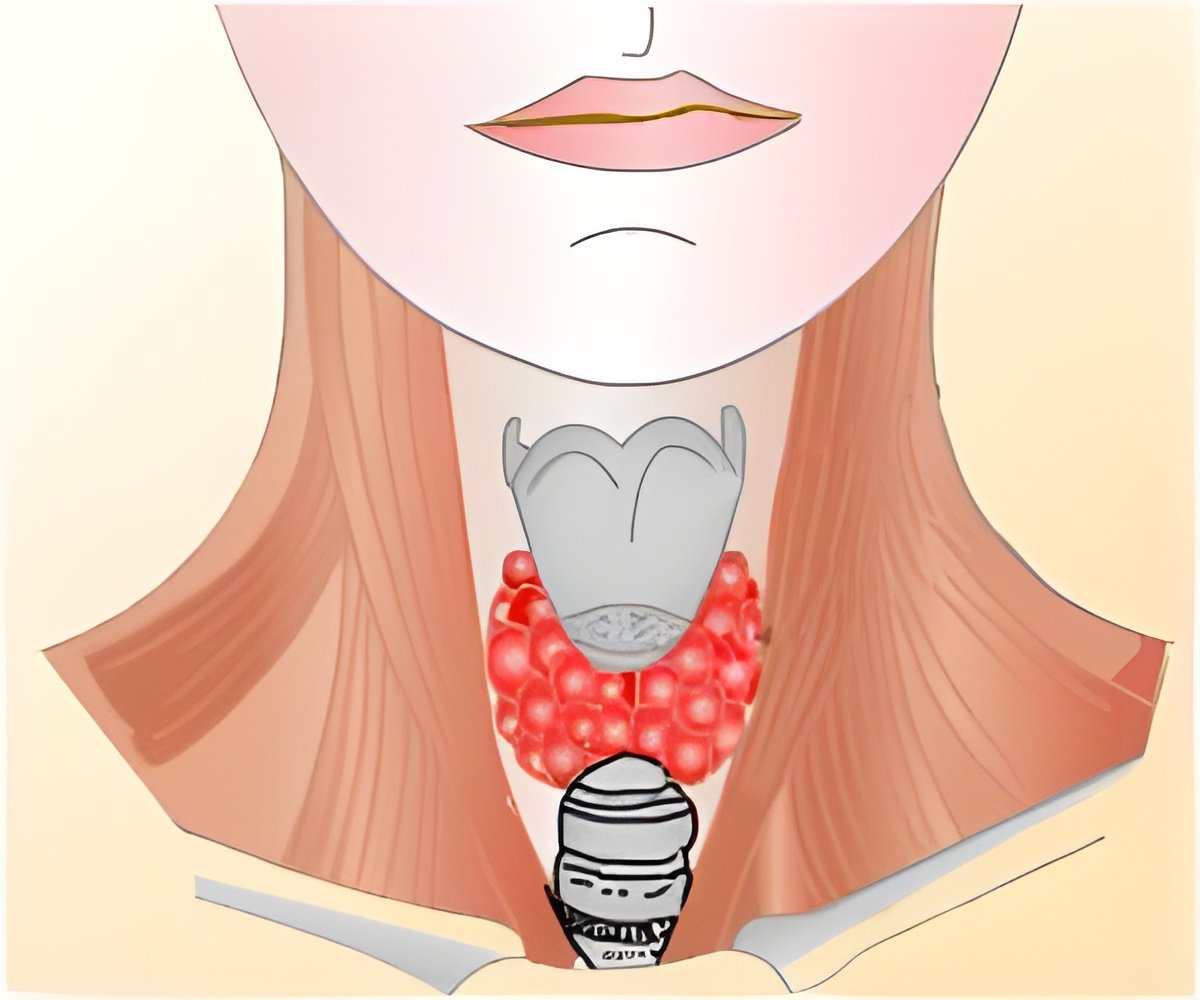
Cometriq is a kinase inhibitor that can block the proteins linked to cancer cell development and growth. The FDA approval was based on the results of a clinical trial involving 330 people with medullary thyroid cancer. Patients who were administered Cometriq survived an average of 11.2 months without tumor growth, compared with an average of four months among the placebo group.
The FDA release added that the benefits and risks must be weighed before prescribing Cometriq as the drug can cause severe and fatal colon bleeding.
San Francisco-based Exelixis markets this drug.
Source-Medindia
 MEDINDIA
MEDINDIA

 Email
Email










