Eli Lilly and Company ( LLY ) has announced the approval by the European Commission (EC) for its radioactive diagnostic agent, Amyvid (florbetapir F 18 Injection),.
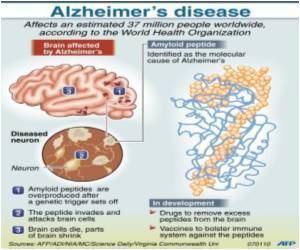
Amyvid had received the approval to be marketed in the U.S in 2012, and now Eli Lilly hopes to introduce it in certain parts of the EU in the next 3 months.
Amyvid is a good bet for the company, as it takes forward its initial objective of developing treatments for Alzheimer's disease. Amyvid promises to be a boon for physicians as it can support diagnostic methods while evaluating patients.
Source-Medindia
 MEDINDIA
MEDINDIA




 Email
Email




