By authorising the use of experimental drugs in the fight against the deadly Ebola virus, the World Health Organisation is allowing a shortcut in drug-vetting procedures that usually take years.
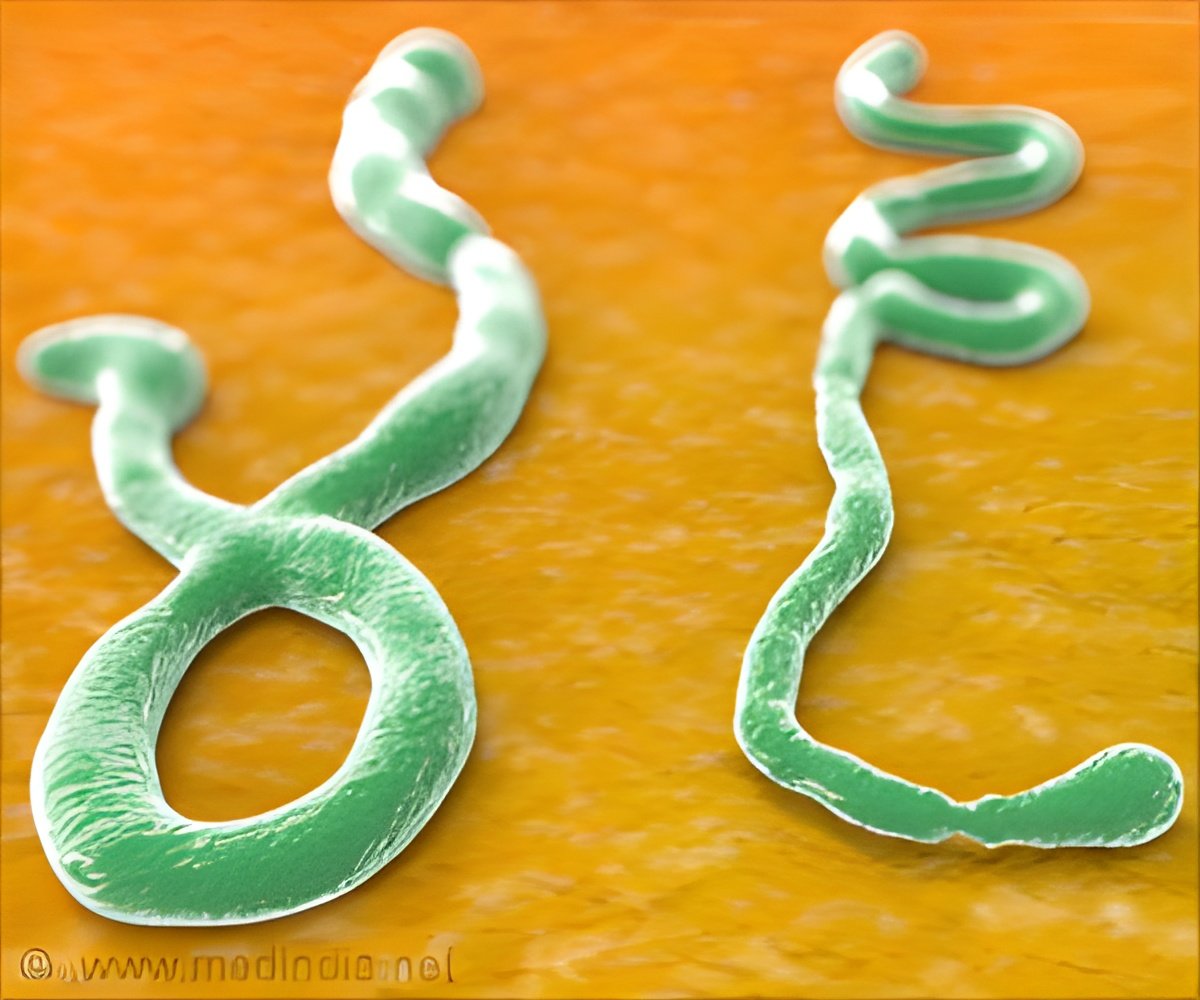
After preliminary tests on animals or cells in petri dishes, a laboratory must obtain permission from health authorities for clinical trials on humans to assess a drug's safety and effectiveness.
This testing usually consists of three phases:
-- Phase one is generally carried out with a sample of fewer than 100 volunteers to assess drug tolerance and check for side-effects.
-- Phase two evaluates the effectiveness of the drug and the optimal dosage, usually on a sample of several hundred people.
-- Phase three compares the treatment either to a dummy drug (placebo) or a reference drug already commercially available, usually with several thousand people. The aim is to confirm a drug's effectiveness, and assess the ratio of effectiveness to tolerance.
For approval, a new treatment must demonstrate a benefit/risk ratio that is equivalent to or better than any existing products.
After a drug has gone to market, it remains under surveillance with an ongoing assessment of any side effects.
If there are any health risks, it can be taken off the shelves at any time.
Source-AFP
 MEDINDIA
MEDINDIA


 Email
Email






